In this article 
View / Download pdf
version of this article
Updated information on Smoking Cessation available from
Smoking Cessation - helping patients stick with it, until they quit bpacNZ, October 2015
Further information on the use of vaping for smoking cessation available from
Smoke and mirrors: is vaping useful for smokers who cannot quit? bpacNZ, August 2018
Key concepts |
- The rate of smoking among New Zealanders is slowly reducing, however more work needs to be done to further reduce
this number
- Brief advice about quitting smoking from a health professional increases the likelihood that someone who smokes will
successfully quit and remain a non-smoker 12 months later
- There is no set manner in which the brief advice to quit needs to be given, although it should be personally relevant
to the patient and describe the benefits to be gained from smoking cessation
- The main groups of pharmacological interventions for smoking cessation are nicotine replacement therapy, bupropion,
nortriptyline and varenicline
|
Advice from primary care helps people who smoke to quit

Smoking has been identified as the major cause of preventable death in OECD countries.1 One of the Ministry
of Health targets for 2010/11 is: “Better help for smokers to quit”.
The results of the 2009 New Zealand Tobacco Use Survey showed an encouraging reduction in the number of people smoking
compared to statistics from previous years. At the time of the survey, 22% of people aged 15-64 years were current cigarette
smokers. When results were first collected in 1986, this proportion was 30% (Figure 1). Although this is only an improvement
of 8% over more than twenty years, it is estimated that each 1% reduction in the population aged 15-64 years who smoke,
represents about 30,000 fewer smokers.2
| Figure 1: Prevalence of cigarette smoking in New Zealanders aged 15 years and over, 1986-2008 (Adapted
from Ministry of Social Development, 2010)1 |
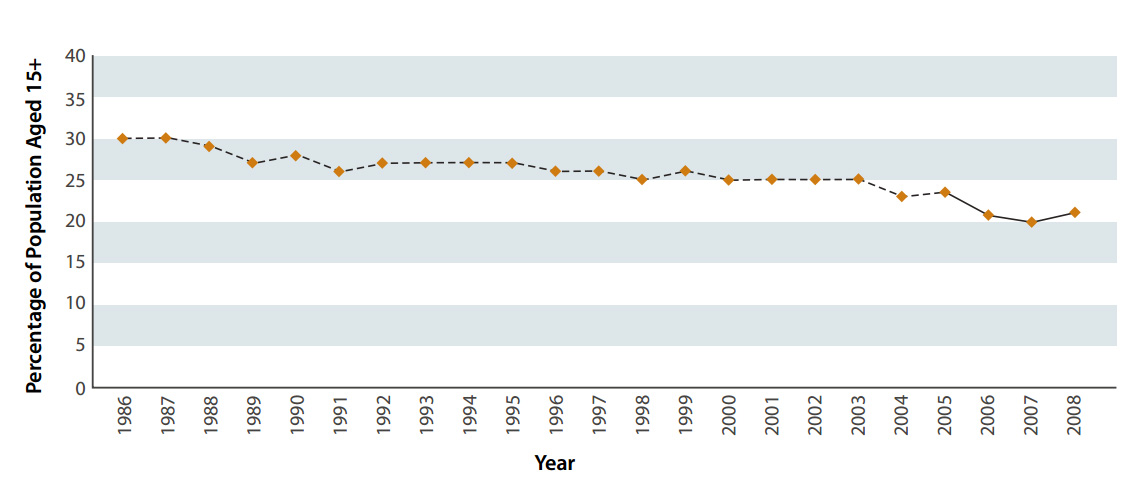 |
Primary care is well placed to help identify smokers and offer smoking cessation advice and referral to smoking cessation
services. There is evidence that brief advice about quitting smoking from a doctor increases the likelihood that someone
who smokes will successfully quit and remain a non-smoker 12 months later.3
PHO Performance Programme goals for smoking cessation
The smoking cessation indicators for the PHO Performance Programme (PPP) are:
- % of the enrolled practice population who have had their smoking status recorded
- % of the enrolled practice population whose most recent smoking status is recorded as “current smoker”
- % of current smokers who have been given brief advice in the last 12 months
- % of current smokers who have been given or referred to cessation support services in the last 12 months
The Programme has identified two data sets that will enable collection and analysis of data for these indicators:
- Recording of smoking status
- Recording the delivery of brief advice and cessation support
Smoking status and, if relevant, smoking cessation advice should be recorded for each patient in their computerised medical
record, using an appropriate code.
Ask, Brief advice, Cessation support
Ask, Brief advice, Cessation support (ABC) has become the standard of care for helping people to quit smoking. The ABC
format can be easily integrated into everyday practice of all health care professionals, so that smokers are presented with
every opportunity to quit.
Nicotine withdrawal
Nicotine use creates a chemical dependency, therefore tobacco users can usually expect to experience withdrawal symptoms
when quitting smoking.
Withdrawal symptoms may include:
- Headache, fatigue, restlessness
- Anxiety, irritability, sleep disturbance, mood swings
- Sweating, dizziness
- Increased appetite,* nausea, stomach cramps
- A craving for more tobacco
* On average, people may expect to gain between four to five kilograms in the first year of abstinence.4 Patients
should be advised to concentrate on achieving and maintaining abstinence from smoking, before managing any weight gain.
A - Ask whether the patient smokes
B - Give brief advice to quit
C - Offer evidence based cessation support
There is no set manner in which the brief advice to quit needs to be given. Most clinicians would agree that the brief
advice should be personally relevant to the patient and describe the benefits to be gained from smoking cessation.
For younger patients it can be helpful to use the incentive that those who quit before the age of 35 years will have a
normal life expectancy. For older patients it can be helpful to remind them that quitting increases life expectancy by reducing
the risk of diseases such as lung cancer, cardiovascular disease and chronic obstructive pulmonary disease.5
Pharmacological interventions to aid smoking cessation
Many people who want to quit smoking will try to do so without any assistance, however, for most smokers quitting is a
difficult process. Smoking cessation advice includes encouragement to quit as well as information about the different pharmacological
treatments available to help.
“Ask about the elephant” smoking cessation e-learning tool
The “Ask about the elephant” programme uses the elephant metaphor to represent that smoking is something
that should not be ignored or go unaddressed.
The tool:
- Provides practical information about ABC and nicotine replacement therapy
- Is endorsed by RNZCGP, awarding CME points
- Allows health professionals to print a certificate as evidence of professional development
- Allows non-prescribing health professionals to register as a Quit Card provider
- Takes 20 - 40 minutes to complete
It can be completed online at: www.smokingcessationabc.org.nz
The main groups of pharmacological interventions available include:
- Nicotine replacement therapy
- Nortriptyline
- Bupropion (Zyban)
- Varenicline (Champix)
Nicotine replacement therapy
To get the most out of NRT:
- Explain what results may be achieved with NRT
- Reassure that NRT is safe
- Use enough NRT
- Use NRT for a long enough time
- Use NRT in a way that best suits the needs of the individual patient
Nicotine replacement therapy (NRT) is safe and cost effective. It can help people quit smoking by reducing the cravings
that are linked with nicotine withdrawal and reduce relapse. It doubles the chances of long-term abstinence regardless of
the amount of additional support provided. Despite this, initiation of NRT remains low and even when it is prescribed, insufficient
duration of treatment and insufficient dosages are common. Poor utilisation rates may result from commonly held misperceptions
about the safety and efficacy of nicotine and NRT. Correct usage is more likely when smokers’ knowledge about the
role of nicotine and the safety and efficacy of NRT is more accurate.6
From September 2009 NRT became available fully funded on prescription (in addition to the Quit card scheme). Different
forms of NRT products are considered equally effective.4
The NRT products currently available in New Zealand are:
- Patches (7 mg*, 14 mg*, 21
mg* per 24 hours and 5 mg, 10 mg, 15 mg per 16 hours)
- Lozenges (1 mg*, 2 mg*)
- Gum (2 mg*, 4 mg*)
- NRT inhaler
- NRT nasal spray
- NRT sublingual tablets (2 mg)
Practice points for NRT use4
- There is a moderate advantage to using a combination of NRT products over just a single product. There are no safety
concerns with combining NRT products.
- NRT should be used for 8 to 12 weeks, but a small number of smokers may need to use it for longer (5% may continue to
use it for up to a year). There are no safety concerns with long-term NRT use.
- NRT is safe to use repeatedly with other attempts to stop smoking by people who have tried to stop but have not succeeded
in the past.
- NRT can be used for “cutting down”, to encourage reduction in cigarettes prior to quitting. Therefore it
is safe to continue smoking while being treated with NRT, initially.
- International evidence shows that NRT is mainly effective in people who smoke ten or more cigarettes per day. However,
it is the person’s degree of dependency (anticipated difficulty in stopping smoking based on their degree of nicotine
dependence, e.g. smoking within thirty minutes of waking) rather than the number of cigarettes they smoke that should be
used to determine whether NRT is likely to be helpful.
- Contrary to product information, the New Zealand Smoking Cessation Guidelines state that it is appropriate for pregnant
women to use NRT.4 The use of NRT in pregnancy carries a small potential risk to the foetus, but NRT is safer
than smoking. Intermittent NRT, e.g. gum or lozenges, should be used in preference to patches. Where the use of a patch
is judged appropriate, the 16-hour patch is preferable.
- Some product information advises patients to wean off NRT patches, however there is no evidence that this is necessary.
People can stop from a full-strength patch straight away, although some people may prefer to slowly reduce their dose.6
- There is insufficient evidence that the use of NRT by young people who smoke improves continuous six-month abstinence
rates. However, expert opinion is that NRT may be used safely by young people (12-18 year of age) who are dependent on
nicotine. N.B. NRT is not recommended for use in young people who occasionally smoke, such as those who smoke in social
situations only.
- NRT can be safely used by people with cardiovascular disease, however, where people have suffered a serious cardiovascular
event, e.g. myocardial infarction or stroke, in the past two weeks or have a poorly controlled disease, treatment should
be discussed with a specialist. Oral NRT products are recommended, rather than longer-acting patches, for such patients.
NRT patches
Most smokers should start on a full-strength patch (21 mg), with the lower dose patches used for weaning-off (although
this is not always necessary). Use of full-strength doses of both 16- and 24-hour patches, for at least eight weeks, have
been found to be more effective than lower strength preparations for people who smoke more than 10 cigarettes per day.4
Patients should be advised to use a new site each day to apply the patch. Some common problems with the patches include
skin reactions, patches falling off, headache or dizziness and sleeping disturbances such as vivid dreams or insomnia.6
NRT gum
To increase compliance with using NRT gum, it is important to carefully explain its use. NRT gum should not be referred
to as chewing gum. The gum should be slowly chewed to release the nicotine, resulting in a hot peppery taste. The gum should
then be parked between the cheek and gums so that the nicotine can be absorbed. After a few minutes, the gum can be chewed
again and then parked (usually in a different area) and the process repeated for 20-30 minutes (although the gum can be
chewed longer than this if desired). NRT gum takes approximately 20 minutes to reach peak concentration. Ten to 15 pieces
of gum per day can be used, hourly if needed. Use is recommended for eight weeks.
Adverse effects reported with the use of the nicotine gum include sore throat, ears and jaw from chewing.6 It
is a good idea to prepare patients for the fact that oral NRT products may be unpleasant initially but will become more
tolerable overtime.
NRT gum is available in 2 mg and 4 mg strengths. In practice, it is usually more effective to use the higher dose. Gum
may also be used in combination with NRT patches.
N.B: Incorrect use, e.g, chewing NRT gum too vigorously, usually results in more nicotine being swallowed, which may cause
irritation to the throat and mouth and hiccoughs.
NRT lozenges
NRT lozenges are sucked and slowly dissolved in the mouth. Nicotine reaches peak plasma concentration in approximately
20-30 minutes. Twelve to 15 lozenges can be used per day, as required.
NRT lozenges are available in 1 mg and 2 mg strengths. As with NRT gum, the higher dose would usually be prescribed. Lozenges
can be used in combination with NRT patches.
NRT inhaler, nasal spray and microtabs
N.B. These products are not currently subsidised.
The NRT inhaler may be used for up to 10-20 minutes every hour. Ten puffs is equivalent to one puff from a cigarette.
Each inhaler cartridge is equivalent to four cigarettes. Patients should aim to use six cartridges per day, although they
may use less than this if their cigarette use prior to quitting was at a lower level. In cold weather, it is advisable to
keep the inhaler warm to help the nicotine vapour be released from the cartridge.
The NRT nasal spray can be used up to hourly, with one spray per nostril.
Microtabs are placed under the tongue and slowly dissolve over approximately 30 minutes. It takes approximately 20 minutes
for the nicotine to reach peak concentration. Hourly use is recommended to achieve the best effect, but the tablets can
be used more frequently if desired. Up to 40 microtabs can be used per day.
 See “Getting the most out of nicotine
replacement therapy” BPJ 20 (Apr, 2009) for further information about prescribing NRT.
See “Getting the most out of nicotine
replacement therapy” BPJ 20 (Apr, 2009) for further information about prescribing NRT.
Bupropion
Bupropion is a dopamine-noradrenaline reuptake inhibitor which became available, fully subsidised, on the Pharmaceutical
Schedule in July 2009. It approximately doubles the chances of long-term abstinence from smoking.4
Seizure risk with bupropion
The recommended dose of bupropion must not be exceeded, as it is associated with a dose-related risk of seizure. The
incidence of seizure at doses up to 300 mg/day is approximately 0.1% (1 in 1000).
A maximum dose of 150 mg daily should be considered for the duration of treatment in patients with pre-disposing risk
factors to a possible lowered seizure threshold, including:7
- Concomitant administration of other medicines known to lower the seizure threshold, e.g. antipsychotics, antidepressants,
antimalarials, tramadol, theophylline, systemic steroids, quinolones and sedating antihistamines
- Excessive use of alcohol or sedatives (also see contraindications)
- History of head trauma
- Diabetes treated with hypoglycaemics or insulin (If the diabetes is poorly controlled use NRT instead)
- Use of stimulants or anorectic products
Bupropion dosing
A quit date should be set for one to two weeks after commencing bupropion. The patient may smoke as normal up to their
quit date and then should stop completely on this date.
- Days 1, 2 and 3: one tablet (150 mg) daily
- Day 4 onwards: one tablet (150 mg) twice a day, with at least eight hours between each dose
- A maximum daily dose of 150 mg is recommended for older people, people with renal or hepatic dysfunction and people
with lowered seizure threshold (see sidebar)
A total course of 120 tablets can be prescribed, but it may be sensible to prescribe a smaller quantity initially so there
is no wastage if the person experiences adverse events or does not manage to achieve abstinence. Patients should be treated
for at least seven weeks but discontinuation should be considered earlier if the patient has not made significant progress
towards abstinence by the seventh week of therapy, since it is unlikely that they will stop smoking during that attempt.8
Contraindications to bupropion use include: seizure disorders (or history of), CNS tumour, abrupt alcohol or sedative
withdrawal, bulimia, anorexia nervosa (or history of), monoamine oxidase inhibitors use within 14 days, lactation.
Insomnia is a very common adverse effect associated with bupropion. Insomnia may be reduced by avoiding dosing at bedtime
(provided there is at least eight hours between doses if an earlier dose is used) or, if clinically indicated, dose reduction.8
Practice points for bupropion use4,8
- There is insufficient evidence to recommend combining bupropion with any other smoking cessation medications
- Bupropion is considered safe and effective for use in people with stable cardiovascular disease
- Bupropion should be used with extreme caution in patients with severe hepatic cirrhosis - the dose should not exceed
150 mg on alternate days
- There is insufficient evidence to recommend the use of bupropion to women who are pregnant or breastfeeding, who smoke
- There is insufficient evidence to recommend the use of bupropion to young people aged under 18 years who smoke
- There is insufficient evidence to recommend the use of bupropion in preventing smoking relapse, i.e. long-term use is
not recommended
Nortriptyline
Nortriptyline is a tricyclic antidepressant that has been shown to be as effective as bupropion and NRT in aiding smoking
cessation.4 However, adverse effects associated with nortriptyline may not be well tolerated in some patients,
e.g. anticholinergic effects (dry mouth, blurred vision) and sedation/drowsiness. The action of nortriptyline in helping
people to stop smoking is independent of its antidepressant effects, therefore it is not restricted to people with a history
of depressive symptoms during smoking cessation.
Nortriptyline dosing
Nortripyline should be commenced while the patient is smoking, with a quit date set for ten to 28 days later. The initial
dose is 25 mg/day, increased gradually to 75-100 mg/day over ten days to five weeks. The maximum dose can be continued for
eight to 12 weeks and tapered down at the end to avoid withdrawal symptoms that may occur if it is stopped abruptly. There
is limited evidence of any benefit of extending treatment past three months.
Practice points for nortriptyline use4
- There is insufficient evidence to recommend combining norptriptyline with any other smoking cessation medication
- People with cardiovascular disease should use nortriptyline with caution, as cardiac conductivity can be affected. Tricyclic
antidepressants are contraindicated in the immediate recovery period after myocardial infarction (MI) and in arrthymias
(particularly heart block).
- There is insufficient evidence to recommend the use of nortriptyline by pregnant women or young people aged under 18
years who smoke
- There is insufficient evidence to recommend using nortriptyline to prevent smoking relapse, i.e. long-term use is not
recommended
Varenicline
Varenicline is now funded with Special Authority, with the following criteria:
- The patient has not used varenicline in the past 12 months AND:
- The patient has had two or more unsuccessful quit attempts using NRT, at least one of which involved comprehensive
advice about the use of NRT
OR
- The patient has had an unsuccessful quit attempt using bupropion or nortripyline
Special Authority is also subject to the patient enrolling in a support and counselling programme for smoking cessation,
including monitoring.
Unsubsidised use of varenicline for a 12-week course costs approximately $700.
Varenicline (Champix) works by binding to nicotine receptors in the brain to reduce the severity of tobacco withdrawal
symptoms, while simultaneously reducing the rewarding effects of nicotine. It approximately doubles to triples the chances
of long-term abstinence (up to 12 months).4
The most commonly reported adverse event is nausea (experienced by approximately 30% of people). In the majority of cases,
nausea occurs early in the treatment period, is mild to moderate in severity, lessens over time and seldom results in discontinuation
of the medication. Varenicline can also cause temporary sleep disturbance and constipation.4
Varenicline has been associated with adverse psychiatric effects. Patients and their families should be advised to monitor
for neuropsychiatric symptoms including changes in behaviour or thinking, anxiety, psychosis, mood swings, agitation, aggression,
depressed mood, suicidal ideation and suicidal behaviour or worsening of pre-existing psychiatric illness. If any of these
symptoms occur, varenicline should be ceased immediately.8
The safety and efficacy of varenicline in people with serious psychiatric illness such as schizophrenia, bipolar disorder
and major depressive disorder has not been established.8
Varenicline dosing
Varenicline is commenced while the patient is still smoking, with a quit date set for one to two weeks later.
- Days 1-3: 0.5 mg, once daily
- Days 4-7: 0.5 mg, twice daily
- Day 8 to end of treatment (12 weeks): 1 mg, twice daily
Patients who cannot tolerate the adverse effects of varenicline may have the dose lowered temporarily or permanently to
0.5 mg, twice daily.
For patients who have successfully stopped smoking at the end of 12 weeks, an additional course of 12 weeks treatment
with varenicline at 1 mg twice daily may be considered. N.B. this additional treatment would not be funded (unless 12 months
had elapsed since starting the first 12-week course).
Practice points for varenicline use
- There is insufficient evidence to recommend combining varenicline with any other smoking cessation medication
- Varenicline is contraindicated in young people aged under 18 years and in women who are pregnant or breastfeeding
- Dose adjustment is not required for older people or people with mild to moderate renal or hepatic impairment. In people
with severe renal impairment, the dose can be reduced to a maximum of 1 mg daily.
- Varenicline has no known clinically meaningful drug interactions
Acknowledgement
Thank you to Dr Nikki Turner, Director, CONECTUS and The Immunisation Advisory Centre, University of
Auckland and Associate Professor Lance Jennings, Virologist, University of Otago, Christchurch and Canterbury
Health Laboratories, Canterbury DHB for expert guidance in developing this article.
References
- Ministry of Social Development. The social report -Cigarette smoking. 2010. Available from: www.socialreport.msd.govt.nz/health/cigarette-smoking.html (Accessed
Nov, 2010).
- Ministry of Health (MoH). Smoking rates track down. Media release. MoH; 2010. Available from www.moh.govt.nz/moh.nsf/indexmh/smoking-rates-track-down (Accessed
Nov, 2010).
- Stead L, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev 2008;2:CD000165.
- Ministry of Health (MoH). New Zealand smoking cessation guideline. MoH: Wellington; 2007. Available from: www.moh.govt.nz/moh.nsf/indexmh/nz-smoking-cessation-guidelines (Accessed
Nov, 2010).
- Thornley S, Jackson G, McRobbie H, et al. Few smokers in South Auckland access subsidised nicotine replacement therapy.
NZ Med J 2010;123(1308).
- The Quit Group. NRT and Quitcards. Available from: www.quit.org.nz/page/providers/research/nrtandquitcards.php (Accessed
Nov, 2010).
- GlaxoSmithKline NZ Ltd. Zyban. Medicine safety datasheet. Available from: www.medsafe.govt.nz (Accessed
Nov, 2010).
- British National Formulary (BNF). 60th Ed. BMJ Group and RPS Publishing: London; 2010.