Age-related macular degeneration is a progressive disease which can lead to diminished visual acuity and loss of central
vision (Figure 1). It is one of the principal causes of blindness. In advanced disease people retain their peripheral
vision but are legally blind due to a loss of central vision.1 Age is a key risk factor, and increases in
prevalence are expected worldwide as populations become progressively older. The development of effective sight-saving
treatments for neovascular (wet) age-related macular degeneration in recent years has improved outcomes for patients,
but has significantly increased ophthalmology resource demands.
In New Zealand, it is estimated that age-related macular degeneration accounts for 48% of cases of blindness among adults
aged 50 years and older, and causes an estimated 400–500 new cases of blindness
per year.2, 3 The prevalence
of age-related macular degeneration in New Zealand is uncertain due to a lack of appropriate studies but it was estimated
in 2014 that it affected 10% of people aged 45 – 85 years, and 38% of people aged over 85 years.4 The risk
of age-related macular degeneration appears to be equal between males and females.5 There are no reliable
estimates of prevalence by ethnicity in New Zealand, however, Māori are known to have higher rates of vision loss from
any cause.2, 4
People with age-related macular degeneration may experience little impact on their daily life in early stages of disease,
but considerable changes in their quality of life, independence and relationships with advanced disease. The rate of progression
is highly variable, but most visual loss occurs once the disease has progressed to “late” age-related macular degeneration
(see below).6 People with age-related macular degeneration have an increased risk of depression and are likely
to experience anxiety about progression of their condition and future vision loss.
Most of the treatment of age-related macular degeneration is carried out by ophthalmologists. Primary care clinicians
have a key role in the identification of patients for referral, counselling patients on preventive measures such as smoking
cessation, a healthy diet and dietary supplements, and assisting patients in managing the effects and psychological sequelae
of their condition.
 |
 |
| Figure 1: Visual changes characteristic of age-related macular degeneration |
Forms of age-related macular degeneration
Age-related macular degeneration progresses through a series of changes in retinal pathology. A patient will not necessarily
have the same stage or type of macular degeneration in both eyes.
 A three minute video illustrating key aspects of the pathogenesis of age-related macular degeneration is
available from: https://youtu.be/qZVPEYIuujo
A three minute video illustrating key aspects of the pathogenesis of age-related macular degeneration is
available from: https://youtu.be/qZVPEYIuujo
Early and intermediate age-related macular degeneration
In the beginning stages of age-related macular degeneration, lipid deposits known as drusen form in the retinal layers
and changes may occur in the retinal pigment epithelium resulting in areas of darkening or lightened pigment. The stage
of disease is defined by the size of drusen deposits in the retina and the presence of retinal pigmentation changes:7
- Early age-related macular degeneration is defined as the presence of medium-sized drusen in one or both
eyes (63 – 125 µm; 125 µm is approximately the size of a large vein at the border of the optic disc).
- Intermediate age-related macular degeneration is defined as the presence of large drusen (>125
µm), or the presence of hypo- or hyperpigmentation in the retinal pigment epithelium.
Early and intermediate stages of the disease may also be referred to as “early/intermediate dry age-related macular
degeneration” due to the absence of exudate or haemorrhage which can occur in neovascular age-related macular degeneration
(known as “wet” age-related macular degeneration; see below).
The risk of progression for patients with early to intermediate age-related macular degeneration is highly variable.
A simple risk score from zero to four can be calculated based on whether the patient has large drusen (one point) or pigment
changes (one point), and whether these are found in one or both eyes, for a total of up to four points (Table
1).
This risk score calculation is not generally used as a clinical tool in New Zealand. In practice, only patients for
whom treatment can be offered would benefit from referral to an ophthalmologist, i.e. those with possible neovascular
(wet) age-related macular degeneration.
Table 1: Early changes in age-related macular degeneration and risk of progression (adapted from AREDS,
2005)8
| Risk score |
Five year risk of developing late age-related macular degeneration* |
| 0 |
0.4 % |
| 1 |
3.1 % |
| 2 |
11.8 – 14.8 % |
| 3 |
25.9 – 35.4 % |
| 4 |
47.3 – 53.1 % |
* For scores of two to four, the risk of future late age-related macular degeneration in one eye varies from the lower
to upper value of the given range, depending on whether late age-related macular degeneration is already present in the
other eye.
Late age-related macular degeneration
The advanced stages of age-related macular degeneration are classified as two forms: geographic atrophy (also
known as “late or advanced dry”) and neovascular (also known as “wet”) age-related macular degeneration.
In some cases both types develop in the same eye.9 Geographic atrophy makes up approximately 80% of cases
of late age-related macular degeneration. Severe vision loss and blindness, however, is more likely to occur in people
with neovascular age-related macular degeneration.9
People with geographic atrophy usually experience a slow and progressive loss of vision: a longitudinal study of people
with geographic atrophy reported that 31% had a three-line loss in visual acuity (equivalent to a symbol needing to be
twice as large for a person to view it) within two years, and 53% within four years.6, 10
People with neovascular age-related macular degeneration can experience a sudden loss or deterioration of vision due
to exudate or retinal haemorrhage. Left untreated, 21% of people have been reported to develop severe vision
loss by six months (greater than six line loss in visual acuity testing), increasing to 42% by three years.10 Neovascular
age-related macular degeneration can be further subdivided into variants such as retinal angiomatous proliferation,
or polypoidal choroidal vasculopathy, which may influence response to anti-VEGF antibodies and treatment decisions
in secondary care.6
Detecting age-related macular degeneration in primary care can be difficult
Early changes in age-related macular degeneration can be detected in a regular eye examination by an optometrist. Adults
are recommended to undergo a general eye examination with an optometrist by the age of 45 years, followed by once every
five years until age 60 years, and once every three years thereafter.11 Patients with visual problems may
require more frequent examination, as appropriate for their condition. Patients with signs of macular degeneration may
be directly referred by the optometrist to an ophthalmology clinic.
General practitioners should enquire whether older patients have had an eye examination recently: for patients who have
not, consider conducting visual acuity testing and direct fundoscopy. Regularly recording
visual acuity will facilitate detection of gradual visual deterioration in older patients. Medical examinations
which older patients require for renewal of their driver licence are a good opportunity to assess all aspects
of vision.
Risk factors for the development of age-related macular degeneration include:
- Age – the condition is rare in people aged 50 years or less1
- Family history – increases odds approximately six-fold5
- Smoking – increases risk approximately 1.9-fold5
- Diabetes – increases risk 1.7-fold5
- Sunlight – risk increases with greater exposure12
- Diets low in fish, fruit and vegetables13, 14
- Previous cataract surgery5
Research into an association between cardiovascular risk factors and prevalence of age-related macular degeneration
has produced inconsistent results. A meta-analysis of relevant studies suggests that risk of age-related macular degeneration
is not significantly altered for patients with high blood pressure, cholesterol or triglyceride levels.5
Symptoms and signs of age-related macular degeneration
Patients with early age-related macular degeneration are typically asymptomatic. Symptoms do not usually occur until
late age-related macular degeneration has developed. The hallmark symptom of late age-related macular degeneration
is a loss or distortion of the central visual field (Figure 1). However, even in late age-related macular degeneration,
patients with changes in one eye only may not notice any alteration of visual acuity or problems with their eyesight.
Symptoms and their rate of progression differ between patients depending on the type of age-related macular degeneration
they have, and include:10
- Difficulty reading fine print, or worsening difficulty extending to larger print
- A dark area in the central visual field at night or in dark environments, which may resolve as vision adjusts to a
lower level of light
- Blurred or wavy vision in the centre of the visual field
- Loss of vision
The most significant sign of age-related macular degeneration is deterioration of best corrected visual acuity. This
visual impairment will not improve with pinhole as would be expected with refractive error, and is often worse with pinhole
as the retinal image is limited to the (affected) fovea. Other signs may be visible on direct fundoscopy, including drusen,
visible as lighter patches in the retina, and retinal haemorrhage or exudates.
 See: “What changes can be seen on direct fundoscopy in patients with different stages of macular degeneration?”.
See: “What changes can be seen on direct fundoscopy in patients with different stages of macular degeneration?”.
The Amsler grid may be useful to assess a distortion of the central visual field, but it is not an essential part of
diagnosis (see: “Amsler grid testing”).
Patients with a gradual loss of visual acuity where age-related macular degeneration is suspected should be referred
to an optometrist.
 Red flags:
Red flags:
Patients with age-related macular degeneration should be urgently referred to an ophthalmologist if they have a sudden
onset distortion or loss of vision.
This may be due to a number of ocular conditions*, including the result of haemorrhage or exudate caused by neovascular
age-related macular degeneration. Even short delays in treatment of a matter of weeks can result in poorer outcomes.1,
10
* There are a number of possible differential diagnoses for a sudden distortion or loss of vision, including diabetic
macular oedema, hypertensive retinopathy, occlusion of the retinal artery, retinal detachment and acute angle glaucoma.15
What changes can be seen on direct fundoscopy in patients with different stages of macular degeneration?
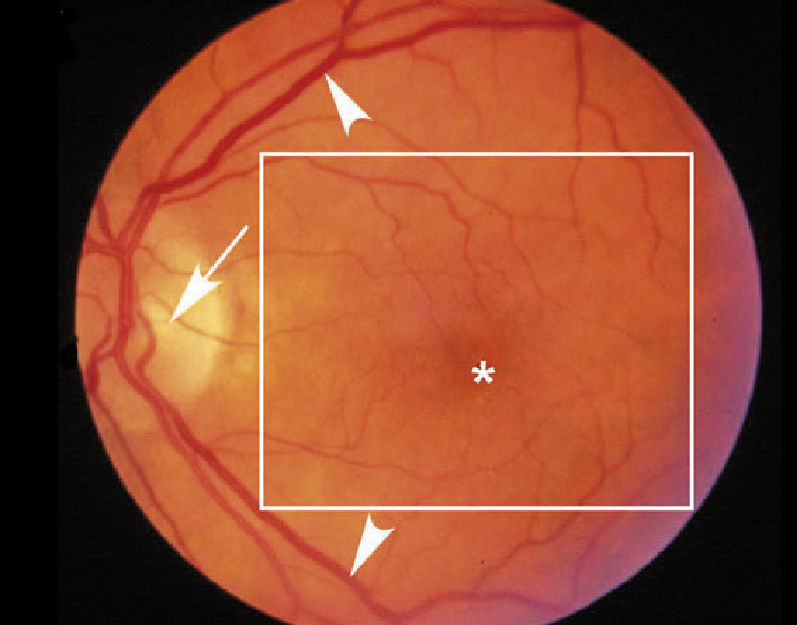 |
Normal eye in an elderly person – retinal blood vessels (arrowheads) are visible emerging
from the optic disc (arrow). These branch into smaller vessels which lead up to the central fovea,
appearing as a darkened circle free of blood vessels (asterisk), approximately the same size as the
optic disc. The extent of the fovea and parafoveal regions which are responsible for high acuity
vision are approximately marked (boxed area).
Image from: http://webvision.med.utah.edu/imageswv/Hagerman.Fig1.jpg |
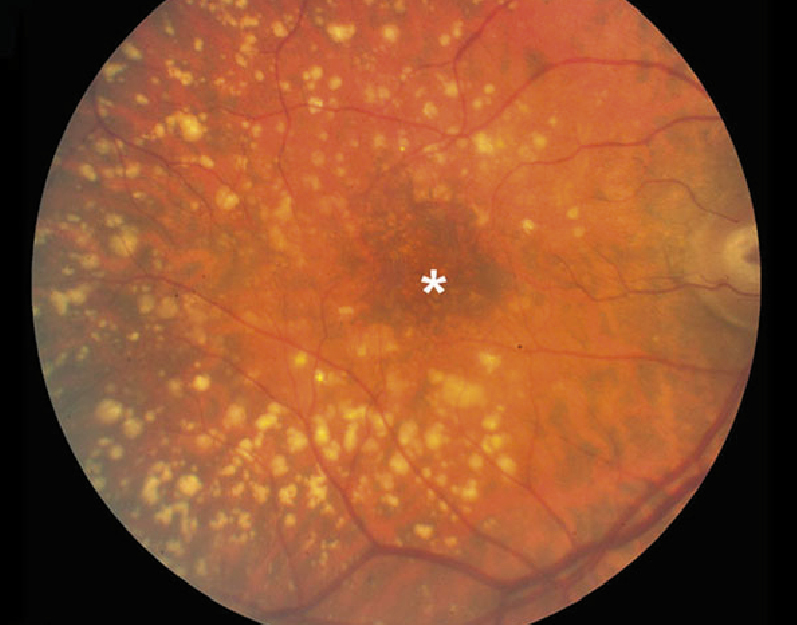 |
Intermediate age-related macular degeneration – in early and intermediate age-related
macular degeneration drusen deposits are visible, appearing as yellow dots. Drusen may be small with
discrete margins or larger with indistinct edges. Changes in retinal pigmentation (darkening or lightening
or the retinal pigment epithelium) may be visible. Haemorrhage and subretinal fluid are absent. In
the image shown, a patient with intermediate age-related macular degeneration has numerous large
drusen (>125 µm in
diameter) in the posterior pole of the fundus, with the fovea largely spared (asterisk)
Image from: http://webvision.med.utah.edu/imageswv/Hagerman.Fig6.jpg |
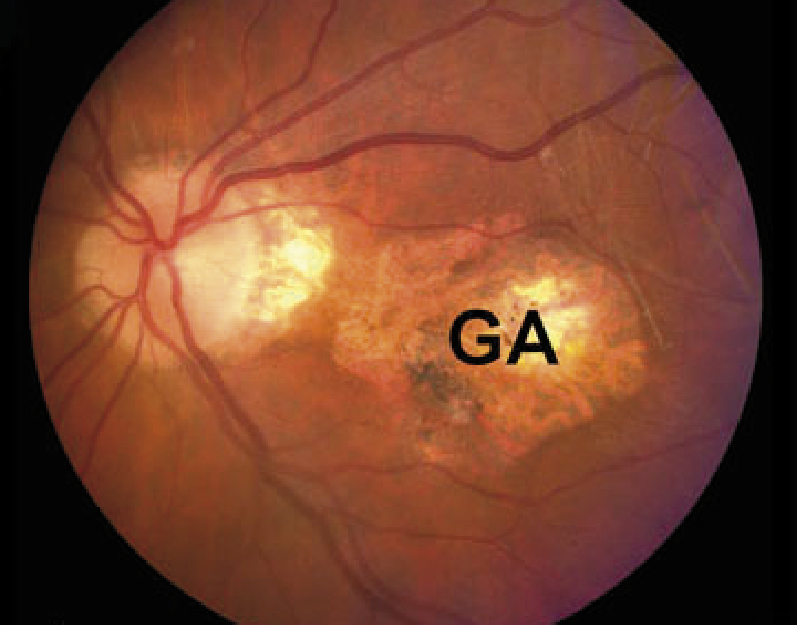 |
Geographic atrophy – drusen deposits accumulate as numerous spots in the retina and areas
of hypopigmentation can be seen which represent atrophy of the retinal pigment epithelium. In advanced
disease, as in this image, the region of atrophy can appear similar to a land mass depicted on a
map (GA), hence the name geographic atrophy. This patient would be expected to have poor central
vision.
Image from:
http://webvision.med.utah.edu/imageswv/Hagerman.Fig7.jpg
|
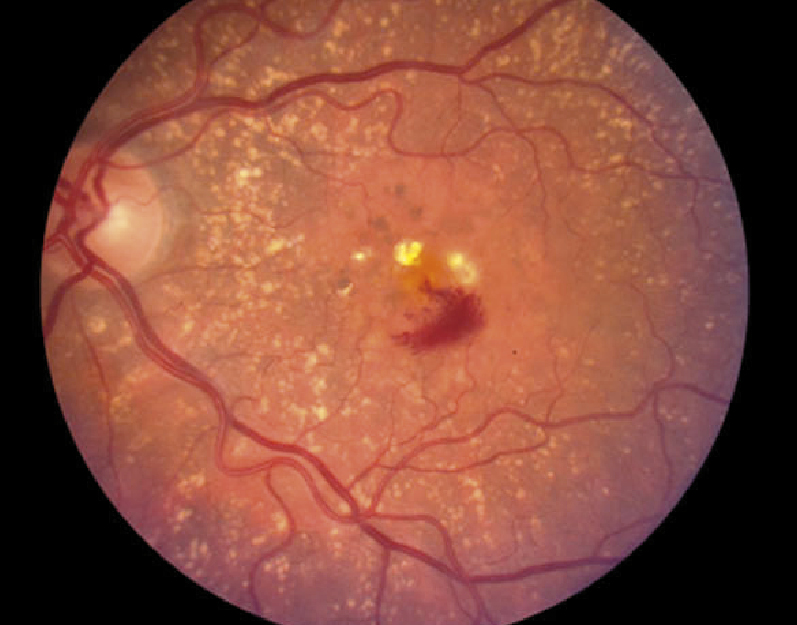 |
Neovascular age-related macular degeneration – new blood vessels break into the neural
retina layer and leak blood constituents, causing accumulation of fluid in the retina and separation
of retinal layers, leading to retinal thickening and scarring. In this image, numerous drusen deposits
are visible throughout the posterior pole of the fundus. Haemorrhage is visible in the macula, and
areas of retinal pigment epithelium atrophy (dark spots in the macula, above and left of the haemorrhage).
Image from: http://webvision.med.utah.edu/imageswv/Hagerman.Fig34.jpg |
Amsler grid testing
The Amsler grid is a tool to assess visual function. It consists of a simple square grid of lines with a central dot,
and is available online, e.g.
http://www.eyecaretrust.org.uk/pdf/eyecare-trust-amsler-chart.pdf.
The Amsler grid can be useful for detecting age-related macular degeneration: patients may see straight lines on the
grid as wavy or blurry. A meta-analysis of 12 studies assessing the performance of the Amsler grid for detecting patients
with neovascular age-related macular degeneration reported a sensitivity of 0.78 and specificity of 0.97.16
The limitations of the Amsler grid are that patients may have already noticed a problem with seeing straight lines or
other changes in vision without the need for a formal test, or may report seeing a normal Amsler grid despite having age-related
macular degeneration due to “filling in” of the visual field; as occurs with the blind spot.17 Clinical guidelines
do not specify its use for diagnosis or monitoring of age-related macular degeneration.10
If clinicians wish to use the Amsler grid, patients should be approximately reading distance from a printout of the
grid, and cover one eye while using the grid to assess each eye individually. Patients should wear any reading glasses
or corrective lenses they normally use.18
Prevention and reducing the risk of progression
The key risk factors for the development of age-related macular degeneration, age and family history, are not modifiable,
but there are steps patients can take to reduce their risk.
Smoking cessation
Smoking cessation is the single most important step patients can take to reduce their risk of developing age-related
macular degeneration or reducing progression.10
 For further information on smoking cessation, see:
www.bpac.org.nz/BPJ/2014/October/smoking-cessation.aspx
For further information on smoking cessation, see:
www.bpac.org.nz/BPJ/2014/October/smoking-cessation.aspx
Avoid UV light
Exposure to sunlight has been identified as a risk factor for the development of age-related macular degeneration. Patients
can be advised to wear UV-blocking sunglasses* when outdoors and to avoid unprotected exposure to UV light (e.g. welding
or UV lamps).12
* advise patients to look for a label which states that lenses block 99-100% of UVB and UVA rays, have UV 400 protection
(blocks rays with wavelengths up to 400 nanometers) or are a lens category three or four.
Include fruits, vegetables and fish in the diet
A varied diet with a range of coloured fruits and vegetables and regular fish intake is likely to reduce the risk of
development or progression of age-related macular degeneration. Lutein and zeaxanthin are carotenoids which form components
of the macula. Dietary sources of lutein and zeaxanthin include egg yolk, corn, kiwifruit, dark green leafy vegetables
such as spinach, lettuce and kale, and various coloured vegetables such as green and orange peppers (capsicum), red grapes,
pumpkin, broccoli, green beans, zucchini (courgette), honeydew melon, apples and oranges.
A meta-analysis of studies assessing dietary lutein and zeaxanthin intake from food sources suggests high intakes protect
against the progression of age-related macular degeneration. People with higher intakes of lutein and zeaxanthin had a
26% reduction in their risk of progression of macular degeneration compared to people with low intakes (relative risk
0.74, 95% CI 0.57-0.97).14 The Women’s Health Initiative study suggests that three and a half servings of
fruits and five servings of vegetables per day (two of which are dark green or orange coloured, or legumes) can provide
approximately 2 mg/day of lutein and zeaxanthin.20
Intake of fish and omega-3 fatty acids, found in oily fish, has also been associated with a reduced risk of age-related
macular degeneration. A systematic review and meta-analysis reported the risk of late age-related macular degeneration
was reduced by 38% with high omega-3 fatty acid intake and by 33% with intake of fish twice per week.21
Supplements may slow progression
A range of supplements available over-the-counter (not subsidised) are advertised as being beneficial to eye health.
These often include herbal products such as flower or berry extracts (e.g. bilberry, marigold or blackcurrant extracts),
fish oils and omega-3 fatty acids, lutein and zeaxanthin, or supplements including a range of vitamins or minerals. There
is evidence that some of these supplements may be beneficial in patients with age-related macular degeneration.
Multivitamin and mineral supplementation
The Age-Related Eye Disease Study (AREDS) and AREDS-2 studies were randomised controlled trials which showed that a
combination of vitamins and minerals can reduce progression in people with early to intermediate age-related macular degeneration.
The AREDS study found that supplementation with a combination of vitamins C and E, β-carotene, zinc and copper reduced
the risk of progression to late age-related macular degeneration by 25% over five years.22 However, due to
safety concerns with the study formulation (in particular, β-carotene, see opposite), a subsequent AREDS-2 randomised
controlled trial was undertaken with a revised formulation:23
- 500 mg vitamin C
- 400 IU vitamin E
- 25 mg zinc
- 2 mg copper
- 10 mg lutein
- 2 mg zeaxanthin
On the basis of these studies patients with early age-related macular degeneration can be advised there is evidence
to support taking supplements similar to the AREDS-2 study supplement formulation*,to reduce the rate of progression.
However, the longer-term safety of this supplementation regimen has not been studied.
N.B. There is no direct evidence to support the use of this supplement formula to prevent the development of early age-related
macular degeneration (primary prevention).
* Based on currently available products in New Zealand, the closest match to the AREDS-2 study formulation would be
to take two tablets per day of Blackmores® Macu-Vision® with one tablet per day of Blackmores® Lutein Defence™ supplements.
The main difference is that this would provide 80 mg of zinc per day instead of 25 mg, but data from the AREDS-2 study
suggest there are no differences in efficacy or safety with this higher zinc dose.23 Other supplements are
available which provide combinations of vitamins and minerals less similar to the AREDS-2 formula or which contain varying
quantities of lutein and zeaxanthin alone.
Lutein and zeaxanthin
The AREDS-2 study assessed lutein and zeaxanthin supplementation as part of a combination of vitamins and minerals.23,
24 On the basis of this study, and a number of smaller studies, supplements containing lutein and zeaxanthin alone
may reduce the risk of progression of age-related macular degeneration, particularly for patients with a low dietary
intake.13
Supplements with no evidence of benefit
Omega-3 fatty acids and fish oils
Although a higher dietary intake of fish and omega-3 fatty acids is associated with reduced risk of age-related macular
degeneration, studies assessing taking omega-3 fatty acids in the form of a supplement have not shown these to offer benefit.13
Bilberry, marigold or blackcurrant extracts
Supplements containing these extracts have not been assessed in clinical trials in patients with age-related macular
degeneration.
Supplements to avoid
β-carotene
β-carotene is an antioxidant which has been promoted as a supplement for improving vision. However, patients are recommended
to avoid supplements containing β-carotene. A study in the 1990s found that supplementation with β-carotene increased
the risk of lung cancer in male current smokers, and the AREDS-2 study observed a higher rate of lung cancer in non-smokers
taking supplements containing β-carotene.24, 25
Management of patients with age-related macular degeneration
While most of the treatment of age-related macular degeneration is performed in secondary care, primary care clinicians
have a vital role in helping the patient adapt to any visual problems they experience.
Treatment in secondary care
Treatment interventions are limited to those patients with neovascular (wet) age-related macular degeneration: the principal
treatment is now intravitreal injections of anti-VEGF antibodies. Other treatments such as photodynamic therapy with verteporfin,
laser ablation of newly formed blood vessels, or surgical approaches have been used in the past but are much less effective
than intravitreal anti-VEGF injections and are now limited to use in very select cases.
Patients with neovascular age-related macular degeneration
Anti-VEGF therapies are now the standard first-line treatment for neovascular age-related macular degeneration worldwide,
and have transformed the management and prognosis of these patients in less than a decade.6, 10 These treatments
not only show high rates of success in stabilising vision (preventing further visual loss in approximately 95% of patients)
but can also improve visual acuity in two-thirds of cases.26, 27 In New Zealand, two anti-VEGF antibodies
which give comparable outcomes are in use: bevacizumab and ranibizumab. The Hospital Medicines List sets criteria for
nationally consistent funded access to these treatments – in most cases bevacizumab would be used first-line and ranibizumab
used if bevacizumab is not tolerated or is not appropriate. Bevacizumab is considerably less expensive than ranibizumab,
and for this reason is the most favoured treatment worldwide. However, this medicine was developed for the treatment of
various cancers and is used off-label for the intravitreal treatment of patients with macular degeneration.1
Patients with geographic atrophy
Treatment options for patients with geographic atrophy are limited. Management focuses on support and counselling, follow-up
monitoring, and advising on measures that may reduce rates of progression (see: “Prevention and reducing the
risk of progression”).10
What patients can expect from anti-VEGF antibody injections
In studies of bevacizumab or ranibizumab for the treatment of neovascular age-related macular degeneration, patients
gain on average approximately three to eight letters in visual acuity (equivalent to one to two lines on a Snellen chart).26
Anti-VEGF antibodies are delivered via intravitreal injection, which are usually given monthly when commenced. The timing
of later injections differ; most ophthalmologists in New Zealand use a “treat and extend” regimen, where a patient is
treated and monitored at each clinic visit, to determine the interval to the next appointment.1 Patients
should expect nine to twelve injections in their first year of treatment, and treatment to last one to two years.
Patients may be understandably hesitant about a treatment which involves injections into the eye. However, the procedure
is carried out with topical, and sometimes subconjunctival, anaesthesia and a 30-gauge needle, and involves minimal discomfort;
rated on average by patients as two on a ten point pain scale (where zero is no pain).28 Approximately half
of patients studied find the actual pain and discomfort experienced is less than anticipated.29 Despite the
treatment burden there is a high level of support and acceptance of continuing injections: in one study of 200 patients
who had received an average of 17.7 injections, 93% reported that they accepted monthly injections due to their importance.30
Patients may experience adverse effects resulting from intravitreal injections: subconjunctival haemorrhage, foreign
body sensation and transient “bubbles” in the inferior visual field (from inadvertently-injected air bubbles) are very
common and the patient should be reassured regarding them. Retinal detachment, retinal/vitreous haemorrhage and damage
to the lens are possible if the needle is not inserted correctly (or if the patient moves). These are uncommon with rates
of 2% or less reported in randomised controlled trials.31 The most serious complication is endophthalmitis
(infectious or sterile), the risk of which can be reduced with meticulous preparation of the ocular surface with povidone-iodine;
published incidence rates are less than 0.1%.32
Living with age-related macular degeneration
Patients with gradual or sudden deterioration in vision can experience changes which impact all aspects of their life,
such as their ability to work, read, use computers, drive, engage in hobbies or sports, and maintain their level of independence
and relationships with others. Patients may be anxious about possible future loss of vision and ultimately blindness,
as well as testing and treatment requirements for their condition. The prevalence of depression in people with age-related
macular degeneration is reported to be between 16–44%.33 Patient reports from the Visual Impairment Charitable
Trust Aotearoa NZ highlight the degree of personal distress for people diagnosed with age-related macular degeneration:3
“Realising that one’s sight is deteriorating and it’s not going to get any better is shattering. It’s traumatic. It’s
an incredible loss.”
“Losing your sight is frightening, really frightening. You lose confidence. You get scared to go out the door. You
need someone to explain the obstacles you might encounter and how to deal with them.”
Patients with visual acuity ≤ 6/24 in the better eye with corrective lenses, or with major visual field defects can
be referred to the Blind Foundation of New Zealand. Patients can learn skills for adapting to life with reduced vision,
and techniques to assist with mobility and orientation. The Blind Foundation helps patients obtain equipment, access financial
assistance and receive peer support and counselling.
 For practical information for patients on managing daily living with sight loss, see:
http://blindfoundation.org.nz/members/useful-resources/handy-hints-for-those-with-low-vision
For practical information for patients on managing daily living with sight loss, see:
http://blindfoundation.org.nz/members/useful-resources/handy-hints-for-those-with-low-vision

Patients with sight loss may experience visual hallucinations
For reasons which are not well understood people with sight loss can experience visual hallucinations, known as Charles
Bonnet syndrome. These range from simple shapes or lines to images of people or buildings. The prevalence of visual hallucinations
in patients with age-related macular degeneration has been reported between 5 – 40%.34
Patients can find hallucinations distressing and be reluctant to mention them to family or medical professionals for
fear they will be labelled as having a psychiatric problem. Patients can be reassured that experiencing these visual hallucinations
is relatively common. Evidence regarding treatment of Charles Bonnet syndrome is limited; in some cases it has resolved
following an improvement in visual acuity with anti-VEGF treatment. Case reports of improvement with olanzapine, tricyclic
antidepressants or donepezil have been published.34
Acknowledgement
Thank you to Dr Logan Mitchell, Consultant Ophthalmologist, Dunedin Hospital, Senior Lecturer, Dunedin School
of Medicine, University of Otago for expert review of this article.