Published: 21st March, 2025 | Updated 27th March 2025
What's changed?
27th March 2025
Table 1 updated to include that colecalciferol 1.25 mg capsules (Multichem Vit D3) are contraindicated by the manufacturer during pregnancy, lactation and in children aged under 18 years.
If you would like to know what changes were made when the article was updated please contact us
Key practice points
- Most people can achieve adequate levels of vitamin D through exposure to sunlight but there are some groups in which this is not the case and supplementation is required
- Testing vitamin D levels (serum 25-hydroxyvitamin D) is not recommended in most cases; empiric supplementation can usually be given based on risk. Clinical scenarios in which testing is appropriate include investigation of possible rickets or osteomalacia.
- Health New Zealand, Te Whatu Ora, published an updated statement in 2024 on vitamin D and sun exposure in pregnancy and infancy in New Zealand. Vitamin D supplementation of pregnant people at risk of deficiency continues to be recommended. New recommendations include:
- Vitamin D supplementation for all exclusively or partly breast-fed infants until age one year (previously supplementation was only recommended for breast-fed infants with specific risk factors for deficiency)
- To consider vitamin D testing for pregnant people with multiple risk factors for deficiency (the dose for vitamin D supplementation for confirmed deficiency is higher than if just at risk)
- If vitamin D supplementation is indicated, prescribe colecalciferol (drops or capsules). Colecalciferol is generally safe and well tolerated at recommended doses.
What’s changed? This is a revision of a previously published article. What’s changed for this update:
- General article revision, including updated dispensing data
- 2024 New Zealand recommendations for vitamin D testing and supplementation during pregnancy and infancy
It has become clearer over time that the proportion of the population who are likely to benefit from vitamin D supplementation is relatively small and mostly restricted to people at risk of, or with, confirmed deficiency. When discussing new or ongoing supplementation with patients who do not have a clear indication for treatment, an evidence-based conversation about the likelihood of benefit may be helpful.
In 2024, Health New Zealand, Te Whatu Ora, published an updated statement for healthcare professionals on vitamin D and sun exposure in pregnancy and infancy.1 A new practice changing recommendation is to offer supplementation for all exclusively or partly breast-fed infants up to age one year. Another new recommendation is to consider vitamin D testing for pregnant people with multiple risk factors for deficiency as this group is more likely to have vitamin D deficiency, and therefore, need higher supplement doses. Vitamin D supplementation continues to be recommended for pregnant people at risk of deficiency, e.g. those living south of Nelson/Marlborough in winter or spring, those with naturally very dark skin or with minimal sun exposure.
Vitamin D dispensing is increasing in New Zealand
The number of patients dispensed vitamin D supplements in New Zealand is increasing over time (Figure 1).2 Over the last five years, there has been a 45% increase in the number of patients dispensed vitamin D supplements. Colecalciferol (vitamin D3) is the predominant formulation dispensed; in 2024, it was the fourth most dispensed medicine in New Zealand, up from 12th in 2016.2
Dispensings across age groups have remained relatively steady between 2020 and 2024 and increase with age (data not shown).2 The highest rate of dispensing is for older patients, particularly those aged ≥ 90 years; in 2024, 511 patients per 1,000 population aged ≥ 90 years were dispensed vitamin D supplements (Figure 2).
The data also show that in 2024, more than one-quarter (262 patients per 1,000 population; 26.2%) of infants aged < 1 year were dispensed vitamin D supplements. It is expected that this number will rise over time as the new recommendation introduced in 2024, to supplement all exclusively and partly breast-fed infants up to age one year, becomes routine practice. Interpretation of this data, however, can be difficult as breast-feeding rates reduce with increased age of the infant and supplementation with vitamin D is not required if infants receive ≥ 500 mL of formula per day. Further analysis of dispensing data in 2024 shows that 243 females per 1,000 population of child-bearing age (i.e. 20 – 49 years) received vitamin D supplementation. People of Indian ethnicity, followed by Other*, European and Asian (excluding Indian) have the highest rate of vitamin D dispensings (Figure 3).2
* Other includes African, Latin American, Middle Eastern and those with unspecified ethnicities

Figure 1. Number of patients per 1,000 population dispensed vitamin D supplements in New Zealand between 2020 and 2024.2

Figure 2. Number of patients per 1,000 population dispensed vitamin D supplements by age in New Zealand in 2024.2

Figure 3. Number of patients per 1,000 population dispensed vitamin D supplements by ethnicity in New Zealand in 2024.2
* Other includes African, Latin American, Middle Eastern and those with unspecified ethnicities
The effects of vitamin D deficiency and possible benefits of supplementation
Vitamin D is understood to affect bone mineralisation by regulating circulating calcium and phosphate.3, 4 If levels of circulating calcium are low, this is corrected through the stimulation of osteoclasts which cause bone resorption via secondary hyperparathyroidism, thereby reducing bone mineralisation.3, 5
Most people with mild to moderate vitamin D deficiency are asymptomatic. Severe deficiency can cause osteoporosis and osteomalacia in adults, and hypocalcaemic seizures and rickets in children.1, 6, 7 Laboratory changes that can occur with vitamin D deficiency (usually severe deficiency) include raised serum alkaline phosphatase, elevated parathyroid hormone (i.e. secondary hyperparathyroidism) and low calcium and phosphate levels.5, 8
 Osteomalacia and rickets. Osteomalacia causes softening of the bones and is characterised by bone pain, muscle weakness and fragility fractures.9 Clinical features of rickets include swelling of the wrists and ankles, leg deformities, delayed tooth eruption and poor growth.7 Vitamin D supplementation can prevent the development of rickets and osteomalacia in at-risk people.10
Osteomalacia and rickets. Osteomalacia causes softening of the bones and is characterised by bone pain, muscle weakness and fragility fractures.9 Clinical features of rickets include swelling of the wrists and ankles, leg deformities, delayed tooth eruption and poor growth.7 Vitamin D supplementation can prevent the development of rickets and osteomalacia in at-risk people.10
 Maternal and neonatal outcomes. Vitamin D deficiency during pregnancy may be associated with adverse neonatal and maternal outcomes including decreased fetal growth rate, low birth weight, gestational diabetes, pre-eclampsia and post-partum haemorrhage; however, further studies are required to establish a causal link.1, 5, 11 Supplementation during pregnancy ensures that the infant is born with sufficient stores of vitamin D to support bone health and prevent rickets.1, 11 Vitamin D may also have a role in preventing gestational diabetes, reducing pre-eclampsia risk, risk of pre-term birth, small for gestational age birth and neonatal and intra-uterine mortality, however, there is insufficient evidence to support these possible benefits and further studies are required.1, 11, 12
Maternal and neonatal outcomes. Vitamin D deficiency during pregnancy may be associated with adverse neonatal and maternal outcomes including decreased fetal growth rate, low birth weight, gestational diabetes, pre-eclampsia and post-partum haemorrhage; however, further studies are required to establish a causal link.1, 5, 11 Supplementation during pregnancy ensures that the infant is born with sufficient stores of vitamin D to support bone health and prevent rickets.1, 11 Vitamin D may also have a role in preventing gestational diabetes, reducing pre-eclampsia risk, risk of pre-term birth, small for gestational age birth and neonatal and intra-uterine mortality, however, there is insufficient evidence to support these possible benefits and further studies are required.1, 11, 12
 Fracture risk. One study has shown reduced fracture risk with combined calcium and vitamin D supplementation in frail older females living in aged residential care but there is little evidence of benefit in people living in the community (see: “Vitamin D in the general population”).11, 13, 14
Fracture risk. One study has shown reduced fracture risk with combined calcium and vitamin D supplementation in frail older females living in aged residential care but there is little evidence of benefit in people living in the community (see: “Vitamin D in the general population”).11, 13, 14
Vitamin D supplementation is generally only beneficial for people at risk of deficiency (see: “Risk factors for vitamin D deficiency”), including all exclusively or partly breast-fed infants up to age one year (see box), or people with confirmed deficiency.1, 6, 11
Although not current practice in New Zealand, revised guidance from the American Endocrine Society (2024) recommends empiric vitamin D supplementation for all children and adolescents aged 1 – 18 years, people aged ≥ 75 years and people with pre-diabetes (see: “Vitamin D supplements may reduce progression of pre-diabetes, mortality and respiratory infection risk”).11 Therefore, the definition of risk for vitamin D deficiency may change over time.
Supplementation is not recommended for the prevention of vitamin D deficiency in people who do not have specific medical conditions or risk factors for vitamin D deficiency (see: “Vitamin D in the general population”).6, 11
New recommendation for infants: Vitamin D supplementation should be considered for all infants who are exclusively or partly breast-fed, initiated as soon as practical but by age four weeks and continuing until age one year.1
Breast milk alone is not likely to contain adequate levels of vitamin D, but it remains strongly supported as the ideal food for infants. Previously, vitamin D supplementation was only recommended in infants who were exclusively or partly breast-fed if they had one or more risk factors for deficiency. The reason for this change is that infants are now exposed to less sunlight due to sun safe practices, and are therefore, more at risk of vitamin D deficiency.1 Infants receiving ≥ 500 mL of formula per day do not require supplementation as infant formula is fortified with vitamin D and intake should be sufficient.1
Vitamin D supplements may reduce progression of pre-diabetes, mortality and respiratory infection risk
There is some evidence that vitamin D supplementation in people with pre-diabetes reduces progression to type 2 diabetes.11 Data from a systematic review and meta-analysis has found that in people with impaired glucose tolerance or pre-diabetes, vitamin D supplementation, in addition to lifestyle modifications for diabetes prevention, reduced the risk of developing type 2 diabetes by 15% or 24 fewer people per 1,000 people, compared to placebo.11 This evidence has led to a recommendation in the 2024 vitamin D guidelines by the Endocrine Society (American) for empiric supplementation of vitamin D in people with pre-diabetes, in addition to lifestyle modification.11
The Endocrine Society (2024) also recommends empiric vitamin D supplementation for: all children and adolescents aged 1 – 18 years to prevent rickets and potentially reduce the risk of respiratory infections; for people aged ≥ 75 years to possibly reduce mortality risk.11 However, evidence is inconsistent and further studies are required.1, 5
Risk factors for vitamin D deficiency
Groups considered to be at high risk of vitamin D deficiency who may benefit from supplementation include:1, 6, 15
- People with naturally very dark skin (this includes many people from South Asia, Africa and the Middle East)
- People with minimal sun exposure due to religious, cultural, personal or medical reasons (e.g. veiled, full coverage clothing, history of skin cancer, taking photosensitive medicines)
- People living in southern regions of New Zealand who spend very limited time outdoors around midday between May and August
- People living in aged residential care facilities
- People with reduced mobility, who are frail or who are house-bound
- Older people admitted to hospital
- People who have had a hip fracture
- People with a medical condition that affects vitamin D or calcium metabolism, e.g. liver failure, renal failure, inflammatory bowel disease, coeliac disease
- People taking certain medicines that affect the metabolism or absorption of vitamin D, e.g. anticonvulsants, rifampicin
- Pregnant people with any of the following:
- Live south of Nelson/Marlborough during winter or spring
- Naturally very dark skin
- Spend very limited time outdoors and/or have minimal sun exposure due to religious, cultural, personal or medical reasons
- Infants with any of the following:
- Exclusively or partly breast-fed receiving < 500 mL of formula
- A sibling who has been diagnosed with rickets or hypocalcaemic seizures (who would in turn already be receiving vitamin D)
- Pre-term or weighed < 2.5 kg at birth
- Naturally very dark skin
- Mother was vitamin D deficient or at higher risk of deficiency
Vitamin D supplementation during pregnancy
Pregnant patients who are at risk of deficiency (listed above) should be prescribed vitamin D.1 New Zealand guidelines also recommend that all pregnant people, who otherwise do not meet criteria for risk, should be advised that they may benefit (and are unlikely to be adversely affected) from vitamin D supplementation, especially during the third trimester, as this is when infant vitamin D stores are predominantly established.1 However, there is inconsistent evidence that vitamin D supplementation during pregnancy improves maternal and neonatal health outcomes (see: “The effects of vitamin D deficiency and possible benefits of supplementation”).1, 11, 12 If vitamin D is taken during pregnancy, it can be continued while breast-feeding.16
Vitamin D testing should be selective
Vitamin D can usually be prescribed to patients who may benefit from supplementation without the need for testing.6, 11 There is no evidence to support vitamin D testing in the general population unless clinical features of deficiency are present.11
There are only certain clinical scenarios where vitamin D testing is appropriate and funded in the community, e.g. investigating possible rickets (or high risk), osteomalacia, disorders of calcium and phosphate metabolism, or sometimes in pregnancy (see: “Testing during pregnancy and infancy”).6, 11 Check with your local laboratory for full clinical criteria. If severe vitamin D deficiency is suspected, also request serum calcium, phosphate, alkaline phosphatase and any other laboratory tests as clinically indicated (e.g. parathyroid hormone for pregnant patients).1, 6
When vitamin D testing is requested, serum 25-hydroxyvitamin D is measured as it is a stable metabolite with a plasma half-life of three weeks.8 A vitamin D serum level > 50 nmol/L is generally considered sufficient, and levels below 25 – 30 nmol/L considered deficient.1, 6 However, the optimal serum vitamin D level is contentious which can make the results of testing difficult to interpret and levels also vary between seasons (may be up to 20 nmol/L difference between winter and summer).8, 17
Testing during pregnancy and infancy
Testing vitamin D levels is not routinely recommended during pregnancy, however, in some cases it may be considered if there are strong reasons to suspect deficiency, because if deficiency is confirmed, a higher vitamin D dose is needed than empirical supplementation for those at risk. New Zealand guidelines recommend that it may be appropriate for pregnant patients with:1
- A history of vitamin D deficiency or insufficiency
- Unexplained laboratory results, e.g. raised serum alkaline phosphatase, low calcium and phosphate
- Unexplained bone pain, unusual fractures or other symptoms or signs suggesting metabolic bone disease
- All of the following risk factors: naturally very dark skin, live south of Nelson/Marlborough during winter or spring, spend very limited time outdoors and/or have minimal sun exposure due to religious, cultural, personal or medical reasons
Vitamin D testing may be considered for infants with hypocalcaemic seizures or an unexplained raised serum alkaline phosphatase.1
Colecalciferol (capsules or oral liquid) is the recommended formulation for patients if vitamin D is indicated (Table 1).6 Hydroxylated derivatives, alfacalcidol or calcitriol, should be prescribed to patients with renal failure, as vitamin D undergoes hydroxylation in the kidney to its active form.16 Dietary calcium intake should be optimised in patients taking vitamin D supplements.6, 11,16
Table 1. Suggested colecalciferol dosing.1, 6, 16, 31 N.B. High intermittent dosing should generally be avoided as it is associated with more adverse effects.11
|
Prevention |
Confirmed deficiency |
Notes |
Adults |
10 micrograms (400 IU) per day (one drop of oral liquid)*; OR
1.25 mg (50,000 IU) every two to three months (one capsule)† |
1.25 mg (50,000 IU) per month (one capsule)†; OR
30 – 40 micrograms (1,200 – 1,600 IU) per day (3 – 4 drops of oral liquid); OR
Contents of one whole bottle of oral liquid per month as a single dose (5 mL)
In moderate to severe deficiency, a loading dose of 1.25 mg (50,000 IU) per day (one capsule)† for ten days may be given, followed by 1.25 mg (50,000 IU) per month (one capsule)† |
Some people with deficiency due to malabsorption states or liver disease may need higher doses |
Pregnancy |
10 – 20 micrograms (400 – 800 IU) per day (1 – 2 drops of oral liquid) |
There is no consensus on the dosing regimen – seek specialist advice; in general, 25 – 50 micrograms (1,000 – 2,000 IU) per day (2 – 5 drops) may be given. 1.25 mg (50,000 IU) per month (one capsule)† may also be considered but safety data for monthly dosing during pregnancy is lacking – and this is an unapproved use.
An individualised treatment programme is required in severe deficiency |
It may be appropriate to continue vitamin D supplementation during breast-feeding
Colecalciferol 1.25 mg capsules (Multichem Vit D3) are contraindicated by the manufacturer during pregnancy and lactation due to a lack of safety data. N.B. This contraindication applies only to this product; colecalciferol is considered compatible with pregnancy and lactation up to doses of 4,000 IU per day. |
Infants and children |
10 micrograms (400 IU) per day (one drop of oral liquid) |
There is no consensus on the optimal dosing regimen – seek specialist advice |
Oral liquid can be given directly into the mouth or applied to the nipple or a bottle teat. The drop can also be added to other food or drink provided that it is all consumed.
For exclusively or partly breast-fed infants, initiate as soon as practical but by age four weeks and continue until age 12 months
Colecalciferol 1.25 mg capsules (Multichem Vit D3) are contraindicated by the manufacturer in children aged under 18 years; dosing regimens for prevention of deficiency are available, e.g. one capsule, every two to three months, but this is an unapproved use. |
* Note the packaging of the currently funded brand of colecalciferol oral liquid (Clinicians) is marketed for use in children, but this product is also suitable for adults, e.g. pregnant people, those with soya or peanut allergy
† The currently funded brand of colecalciferol capsules (Multichem Vit D3) contains soya oil and will not be suitable for patients with an allergy to soya or peanut.16 Ingredients of animal origin, including the gelatin capsule, are halal compliant.16
Refer to the NZF for information on cautions and contraindications, and dosing information for alfacalcidol and calcitriol: https://nzf.org.nz/nzf_5370
Multivitamins containing colecalciferol can be purchased over-the-counter (OTC) or one product is available funded on prescription. Vitamin D-only supplements (without other vitamins/minerals) are also available OTC. Multivitamin preparations often do not contain sufficient quantities of vitamin D (or the required dose would exceed levels of other vitamins, e.g. vitamin A), therefore, should not be recommended in place of prescribed colecalciferol for patients needing vitamin D supplementation. Some patients may already be taking OTC supplements containing vitamin D; check the packaging to see if the dose is sufficient. If the dose is not adequate, discuss with the patient that it would be preferable for them take prescribed colecalciferol to meet the recommended daily dose. If the patient would prefer to continue taking the OTC supplement, prescribe a ‘top up’ dose of colecalciferol if needed.
Adverse effects of vitamin D supplementation have been reported
Vitamin D supplements taken at the recommended dose are generally well tolerated and safe.18, 19 However, adverse effects and toxicity (hypervitaminosis D) are possible with long-term use of excessive daily doses (e.g. > 4,000 IU/day), e.g. hypercalcaemia, decreased bone mineral density, increased fracture and falls risk.18–20 Reports of toxicity are increasing, due in part to increasing prescribing and use of OTC supplements, e.g. multivitamins.18
Clinical features of severe hypercalcaemia include:6, 20
- Confusion
- Apathy
- Polyuria
- Polydipsia
- Reduced appetite and associated anorexia
- Vomiting
- Abdominal pain
- Constipation
- Muscle weakness
- Urolithiasis
Vitamin D in the general population
People derive approximately 80% of their circulating vitamin D from the ultraviolet B (UV-B) rays in sunlight, therefore, most people can achieve adequate vitamin D levels by spending time outdoors during the day. Skin needs to be exposed to direct sunlight to allow the synthesis of vitamin D to occur, as glass blocks UV-B rays.21
Vitamin D is also absorbed from a small amount of foods and vitamin D-fortified products, but this is only a minor source of vitamin D (see: “Diet is a minor source of vitamin D”).6, 21 Figure 4 shows the metabolic pathway of vitamin D.
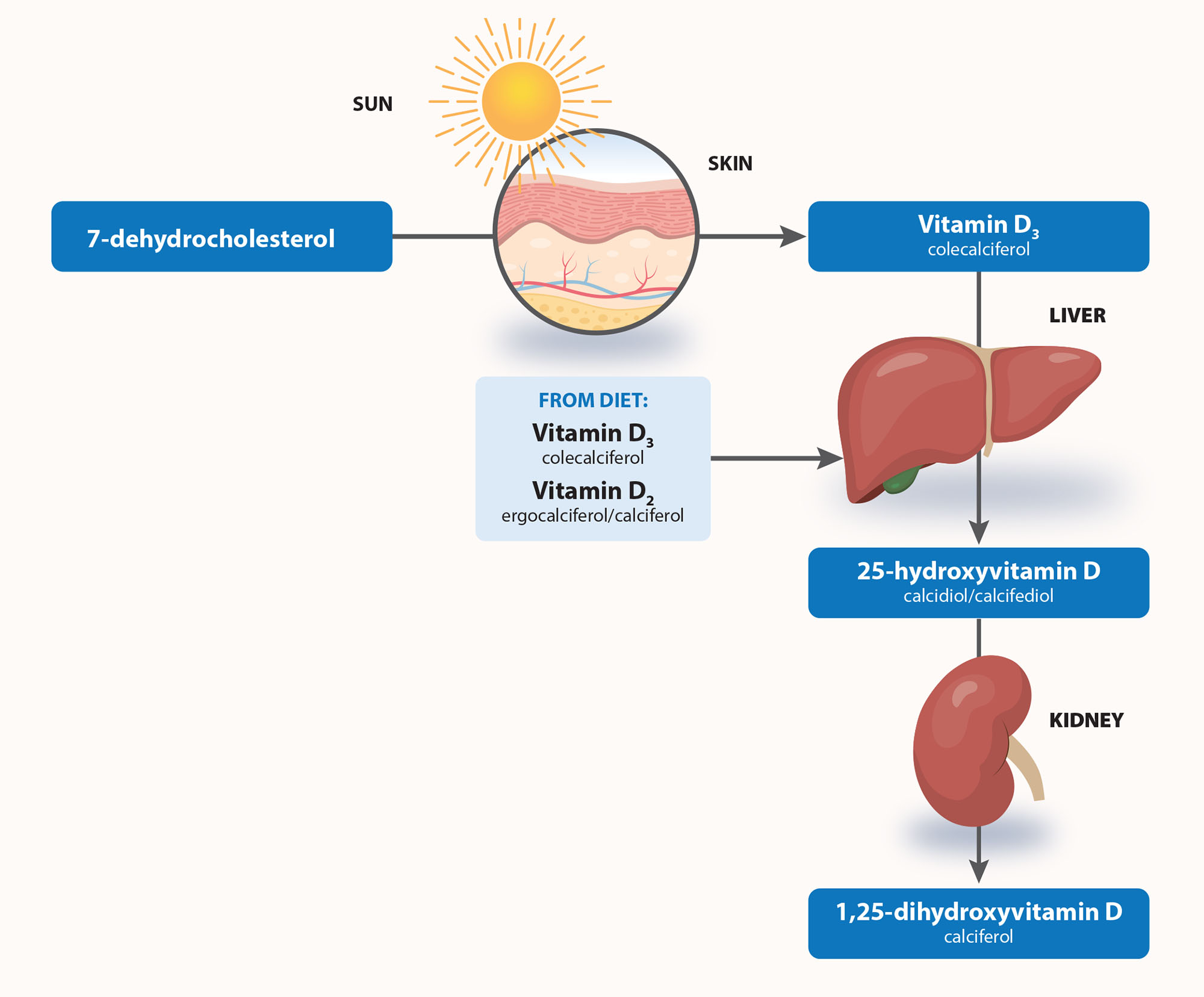
Figure 4. Metabolic pathway of vitamin D. Adapted from Glendenning, 2015.23
Practice Point: Application of sunscreen is still strongly recommended when exposed to sunlight. There is currently limited evidence that sunscreen use negatively affects vitamin D status although high SPF sunscreens that are now recommended and widely used have not been included in studies.6, 22
How much sunlight is enough?
The recommended intake of vitamin D is between 200 and 600 IU per day.24 The optimal duration of sun exposure in New Zealand to maintain adequate vitamin D levels depends on the location and time of year (vitamin D levels significantly vary with seasons)6:
- A walk or other form of outdoor activity most days in the early morning or late afternoon from September to April with face, arms and hands exposed is recommended6
- At the height of summer, only six to eight minutes of sun exposure may be sufficient to produce 1,000 IU of vitamin D8, 24
- During winter or an overcast day, a greater length of time should be spent outdoors, e.g. 30 – 50 minutes, as fewer UV-B rays reach the skin8, 21
Darker skin pigmentation (i.e. higher levels of melanin) is associated with lower rates of vitamin D production; people with naturally dark skin may require three to six times more sun exposure to achieve equivalent levels of vitamin D production.8, 21
N.B. It is not possible to develop vitamin D toxicity due to exposure to sunlight.6
Time required to maintain adequate vitamin D levels has been estimated in Australia for people with different skin types. See Appendix 1A in Position statement: balancing the harms and benefits of sun exposure (2023), available from: https://espace.library.uq.edu.au/view/UQ:17c76bd. Interactive tables are also available here. This information can be generally applied to people living in New Zealand by choosing a city with a similar latitude, e.g. Melbourne for Auckland, Hobart for Christchurch.
The use of sun beds to boost vitamin D levels is not recommended.6 Sunbed use is associated with an increased risk of melanoma, which rises with greater use and earlier age of first use.6 The risks of skin damage and melanoma must be balanced against the risk of vitamin D deficiency. In addition, sunbeds primarily deliver UV-A rays, whereas UV-B is needed for vitamin D synthesis.
Diet is a minor source of vitamin D
Very few foods naturally contain vitamin D and it is suggested that diet (vitamin D-fortified and vitamin D-rich foods) only contributes approximately 10 – 20% of a person’s vitamin D requirement.15, 25 Diet can, however, be an additional source of vitamin D during winter months or when sun exposure is reduced. Vitamin D-rich food sources include cod liver oil, tuna, oily fish (e.g. sardines, salmon), and to a lesser extent, egg yolk and some mushrooms.15, 26 Vitamin D-fortified foods are not as widely available in New Zealand as in some other countries, but include some margarines, yoghurts, plant-based alternate milks and breakfast cereals. For information on the vitamin D content in most foods in New Zealand, see: https://www.foodcomposition.co.nz/foodfiles/concise-tables/.
Research has confirmed that vitamin D supplementation for the general population provides no net benefit:27
- A 2018 meta-analysis including 81 randomised controlled trials found vitamin D supplementation did not have a clinically meaningful effect on bone mineral density, fractures or falls10
- The 2017 New Zealand-based Vitamin D Assessment (ViDA) study found that high-dose colecalciferol supplementation (100,000 IU per month for two to four years) did not prevent fractures or falls in healthy people (mean age 66 years)28
- A 2017 meta-analysis including over 50,000 participants concluded that vitamin D (with or without calcium) did not significantly affect fracture risk in older adults living in the community29
- The United States Preventive Services Task Force (2018; currently under revision):30
- Found insufficient evidence to recommend for or against vitamin D and calcium supplements (alone or in combination) in males and pre-menopausal females for the prevention of fractures
- Recommends against daily supplementation of both vitamin D (≤ 400 IU) and calcium (≤ 1,000 mg) for the primary prevention of fractures in post-menopausal females living in the community
- Found insufficient evidence to assess the benefits and risks of daily supplementation of both vitamin D (> 400 IU) and calcium (> 1,000 mg) for the primary prevention of fractures in post-menopausal females living in the community
Inconclusive research has created confusion
There are various health claims associated with vitamin D supplementation, but a lack of evidence to support them. This has created confusion, both for patients trying to make informed decisions and for clinicians in guiding their care.
Numerous observational studies have examined associations between vitamin D deficiency and a range of medical conditions, including cardiovascular diseases (e.g. myocardial infarction, stroke), cancer, autoimmune, inflammatory and infectious diseases.5, 11, 14 However, a causal link has not been established.5, 11, 14 It may be more likely that low vitamin D is a marker of reduced health, rather than a cause.
