
B-QuiCK: Prescribing testosterone in ageing males
Assessing older males with potentially low testosterone levels
- Only investigate testosterone levels in patients with specific symptoms of hypogonadism that are impacting their quality of life (for symptoms and signs of hypogonadism, click here)
- Consider factors that may influence testosterone levels such as co-morbid medical conditions (including mental health) or prescribed medicines (for examples, click here), lifestyle and psychosocial factors and testosterone supplementation
- Address these before progressing, if identified
- Perform a clinical examination including:
- Assessment of body hair distribution, testicular size, breast tissue (ask about any recent changes)
- Measure height, weight, waist circumference
- Calculate body mass index (BMI)
- Perform a digital rectal exam
Investigate testosterone levels only if significant features of hypogonadism are present
A suggested approach to the investigation of serum testosterone levels and treatment initiation in patients with clinical features of hypogonadism. N.B. Reference ranges for testosterone differ depending on the immunoassay used; consult with your local laboratory.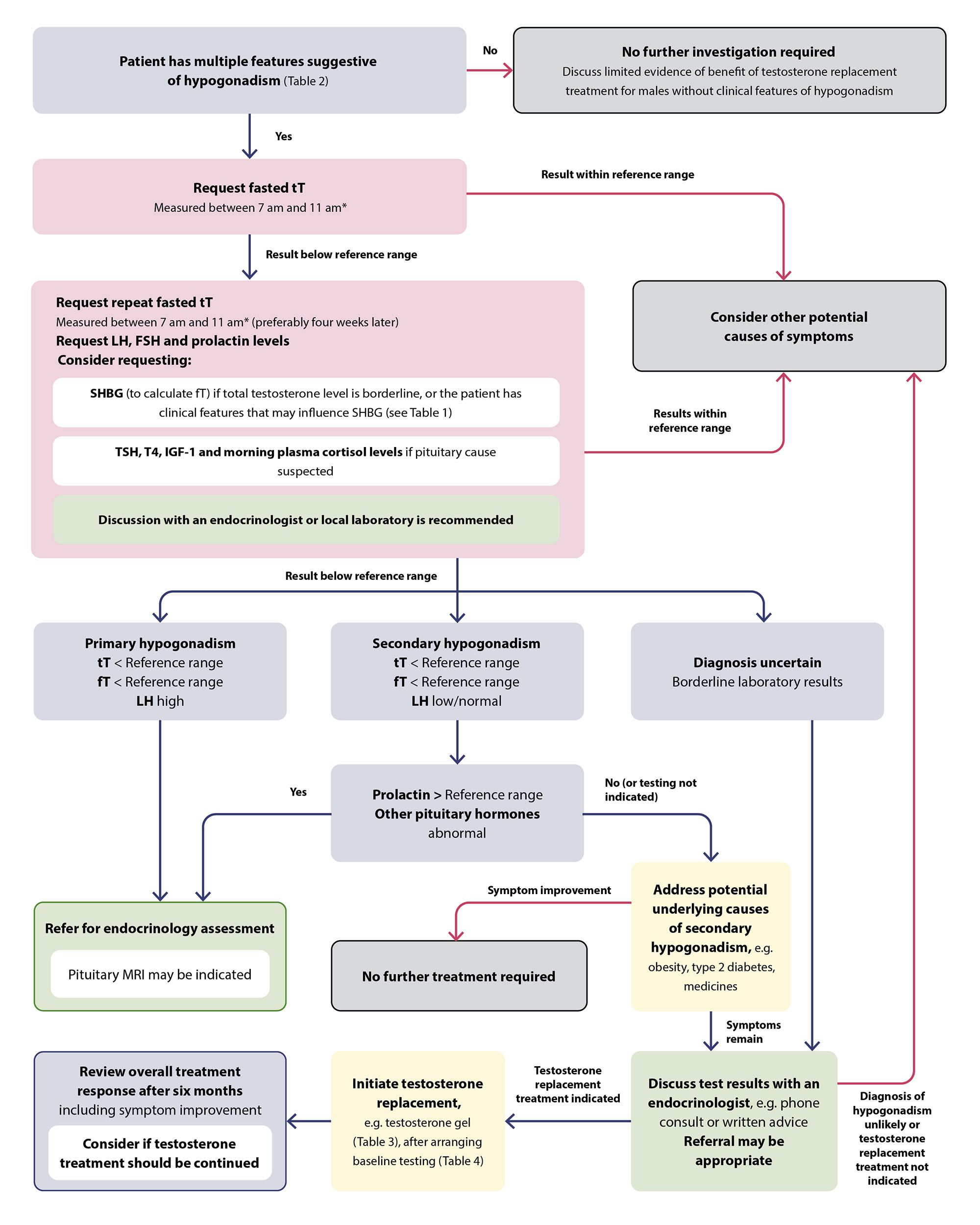
* Measure total testosterone in a shift worker two to three hours after waking
FSH = follicle-stimulating hormone; fT = free testosterone; IGF-1 = insulin-like growth factor-1; LH = luteinising hormone; SHBG = sex hormone-binding globulin; TSH = thyroid stimulating hormone; tT = total testosterone; T4 = thyroxine.
- The threshold for hypogonadism diagnosis and initiating testosterone treatment is not clear but should be low, i.e. < 8 nmol/L (local expert opinion)
- Consider discussing patients with borderline (i.e. 8 – 12 nmol/L) testosterone levels or inconclusive results with an endocrinologist
Managing older males with established testosterone deficiency
Only initiate testosterone replacement treatment in symptomatic patients with laboratory-confirmed hypogonadism on two separate occasions
Testosterone contraindications:
- Males who wish to remain fertile
- Metastatic prostate cancer (or palpable prostate nodule or induration)
- Elevated prostate-specific antigen (PSA)
- Elevated haematocrit
- History of liver tumours or breast cancer
- Hypercalcaemia
- Severe or poorly controlled congestive heart failure
For cautions, click here
Before prescribing testosterone:
- Address any modifiable causes, e.g. switch, reduce or deprescribe medicines associated with hypogonadism (for examples, click here)
- Recommend non-pharmacological interventions and lifestyle changes, if appropriate, e.g. weight loss, stress reduction
- Request baseline testing (for recommended investigations, click here)
- Discuss adverse effects, monitoring requirements, the uncertain long-term risks and need for life-long treatment with the patient
Prescribing testosterone:
- Transdermal testosterone gel is the preferred formulation and should be trialled for six months (for dosing information, click here)
- Arrange patient follow up at 3, 6 and 12 months and then annually, thereafter, to evaluate clinical response and adverse effects
- Beneficial effects may not be apparent for at least three months. N.B. Maximal therapeutic effect may not be obtained until 12 months of treatment.
Potential adverse effects of testosterone:
- Secondary polycythaemia (increases risk of cardiovascular and thrombotic complications e.g. venous thromboembolism)
- Prostate enlargement causing, or worsening existing lower urinary tract symptoms
- Acceleration of previously undetected (or existing) prostate cancer
- Reduced sperm production
- Changes in mood and behaviour
This list is not extensive, click here for more information
Recommended monitoring includes:
- Serum testosterone levels – titrate dosing gradually to a testosterone level where symptom improvement occurs but avoid high-normal levels in older patients
- PSA – an increase > 1.4 ng/mL over a 12-month period following initiation of testosterone should be discussed with a urologist
- Full blood count – stop testosterone in patients with CVD risk factors whose haematocrit increases > 54%
- Dose reduction or switching formulation may be appropriate in those with a low CVD risk (instead of treatment cessation)
Switching to a different formulation could be considered once the dose has been stabilised and evidence of benefit is established
Discontinue treatment if adverse effects are intolerable or severe, or if no clinical benefit is observed within six months
