Published: June, 2024 | Review date: June, 2027
The purpose of this audit is to assess anticholinergic burden in
older patients who are prescribed anticholinergic medicines
and determine if their current treatment is still appropriate.
Anticholinergic medicines are indicated for range of medical
conditions and include antidepressants, antihistamines,
antipsychotics and medicines to treat urinary urgency and
incontinence (Table 1). People who are prescribed multiple
medicines with anticholinergic activity are at increased risk
of adverse effects (e.g. dry mouth, blurred vision, urinary
retention and drowsiness) and this cumulative anticholinergic
influence is referred to as the anticholinergic burden,
although it can occur with just one anticholinergic medicine.
In New Zealand, more than 40% of people aged over 65 years
are exposed to medicines with anticholinergic activity.1
This
group is more likely to experience anticholinergic burden
due to age-related changes in physiology, and increased
likelihood of multiple morbidities requiring management
with anticholinergic medicines. To prevent unnecessary
exposure to associated risks, anticholinergic medicines
should only be prescribed to people with a specific clinical
indication for treatment, and at the lowest effective dose for
the shortest period of time.
Given the range of medical conditions that anticholinergic
medicines are prescribed to manage, each clinician is likely
to have a number of patients who are being treated with
these medicines; some for longer than is recommended or
with a higher dose than is necessary. These patients may
benefit from a dose reduction or deprescribing, depending
on the clinical scenario and the patient’s therapeutic goals
and treatment preferences. Patients who still require
pharmacological management may benefit from switching
to medicines with lower or no anticholinergic activity, if
available. Non-pharmacological interventions should also be
prioritised to reduce the required dose of, or overall need for,
anticholinergic medicines.
When deprescribing or switching medicines, gradual dose
tapering of the original anticholinergic medicine may be
required to limit withdrawal symptoms. A general “rule of
thumb” for tapering anticholinergic medicines is to reduce
the prescribed dose by 25 – 50% over a period of one to four
weeks. Close monitoring is required over the tapering period
for anticholinergic withdrawal symptoms (over the first one
to three days) and recurrence of symptoms associated with
the condition originally being treated (after approximately
seven days). Patients who develop withdrawal symptoms or
a reoccurrence of their original symptoms should restart the
medicine at the lowest tolerated dose and reattempt a slower
tapered reduction after 6 – 12 weeks. Alternate day dosing
may be beneficial in situations where available medicine
strengths are not appropriate for tapering. Clinicians should
ensure that any anticholinergic medicines prescribed for
short-term symptom management are not inadvertently
continued, e.g. orphenadrine for muscle spasms associated
with a lower back strain, promethazine for nausea and
vomiting or motion sickness.
There will be some patients taking anticholinergic medicines
long-term for whom reducing the dose or stopping the
anticholinergic medicine is not appropriate, e.g. clozapine
for schizophrenia. If required, medicines with anticholinergic
activity should be prescribed at the lowest effective dose, for
the shortest possible duration.
For further information on anticholinergic burden in
older people, see: bpac.org.nz/2024/anticholinergic.aspx
- Nishtala PS, Narayan SW, Wang T, et al. Associations of drug
burden index with falls, general practitioner visits, and mortality
in older people. Pharmacoepidemiology and Drug 2014;23:753–8. doi:10.1002/pds.3624.
Table 1. Examples of prescription and over-the-counter medicines with anticholinergic activity.
N.B. This list is not exhaustive and
should be used a general guide only as there is inconsistency in anticholinergic rankings between sources. Any medicine with any level of anticholinergic
activity should be used with caution in patients susceptible to the adverse effects, especially if used in combination.
| Class |
Medicines with anticholinergic activity |
| High anticholinergic activity |
Mixed evidence for high anticholinergic activity* |
Moderate to low anticholinergic activity |
Antidepressants
SSRIs and SNRIs |
|
Paroxetine |
Citalopram, escitalopram, fluoxetine, sertraline, venlafaxine |
TCAs and other |
Amitriptyline, clomipramine, imipramine |
Nortriptyline |
Dosulepin, mirtazapine, moclobemide |
Antiepileptics |
|
|
Carbamazepine |
Antihistamine |
Chlorphenamine, dexchlorpheniramine, diphenhydramine, doxylamine, promethazine |
|
Cetirizine, fexofenadine, loratadine |
Antinausea |
Meclozine (meclizine) |
|
Cyclizine, prochlorperazine |
Antipsychotics |
Chlorpromazine, levomepromazine |
Clozapine, olanzapine, quetiapine |
Amisulpride, aripiprazole, haloperidol, lithium, risperidone, ziprasidone |
Benzodiazepines |
|
Alprazolam |
Clobazam, clonazepam, diazepam, lorazepam, oxazepam, temazepam |
Bronchodilators (antimuscarinic) |
Ipratropium |
|
Glycopyrronium, tiotropium, umeclidinium |
Cardiac medicines |
Atropine |
Digoxin |
|
Diuretics |
|
Furosemide |
|
Gabapentinoids |
|
|
Gabapentin, pregabalin |
Gastrointestinal medicines |
Hyoscine (scopolamine) |
|
Domperidone, loperamide, metoclopramide |
Skeletal muscle relaxants |
Orphenadrine |
|
|
Opioids |
|
|
Codeine, dihydrocodeine, fentanyl, methadone, morphine, oxycodone, pethidine, tramadol |
Parkinson’s medicines |
Benzatropine, procyclidine |
|
Amantadine, levodopa |
Urinary urgency and incontinence medicines |
Oxybutynin†, solifenacin |
|
|
SNRI = serotonin and noradrenaline reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant
*High anticholinergic activity according to some, but not all anticholinergic rating scales
†The majority of evidence suggests oxybutynin has high anticholinergic activity
Summary
This audit identifies patients aged 65 years and over who are
currently taking an anticholinergic medicine to assess their
anticholinergic burden, whether the indication for treatment
remains and if reducing the dose (or stopping or switching
the medicine) is appropriate.
Recommended audit standards
Ideally, all patients who have been taking an anticholinergic
medicine for longer than six months* should have undergone
an assessment of their anticholinergic burden and have
documented evidence in their patient record of an indication
for ongoing treatment or evidence of a discussion about
stepping down to a lower dose or stopping completely. This
audit identifies patients who are prescribed an anticholinergic
medicine and should have their anticholinergic burden
assessed.
*The recommended duration of treatment for anticholinergic medicines
varies depending on the condition being managed. Six months has
been selected pragmatically for this audit to allow sufficient time for
patients who are prescribed anticholinergic medicines, e.g. oxybutynin
and solifenacin to treat urinary urgency and incontinence, to have
experienced an improvement in symptoms.
Eligible patients
All patients aged 65 years or over who are prescribed at least
one anticholinergic medicine are eligible for this audit.
Identifying patients
 This is a “working audit” where the data sheet is filled in over
time when you have a consultation for any reason with an
eligible patient until the required number of patients has
been reached.
This is a “working audit” where the data sheet is filled in over
time when you have a consultation for any reason with an
eligible patient until the required number of patients has
been reached.
Sample size
The number of eligible patients will vary according to your
practice demographic. For the purposes of this audit, continue
until at least ten eligible patients have been identified and
included.
Criteria for a positive outcome
Anticholinergic burden should be assessed in a patient who
is prescribed an anticholinergic medicine for longer than
six months. If there is no record of a recent anticholinergic
medicine assessment in the patient’s clinical notes, this
should be undertaken at the time or planned for their
next appointment – the audit entry for the patient should
remain open until this is completed. The assessment
should include evaluation of all anticholinergic medicines
currently prescribed to the patient, any over-the-counter
use of anticholinergic medicines, and any adverse effects
they are experiencing that could be related to their use of
anticholinergic medicines (e.g. falls).
Based on the results of the assessment, a decision should
be made to continue prescribing the anticholinergic
medicine because the patient has an ongoing indication
and is benefiting from treatment, or that the patient would
benefit more from reducing or stopping (or switching)
the anticholinergic medicine because symptom relief is
insufficient, or they are experiencing adverse effects.
Following the assessment, a positive result is achieved if the
patient’s clinical notes contain:
- Documented evidence of assessment of anticholinergic
burden in the last six months (either undertaken
previously or as part of this audit); AND EITHER
- A record of a current indication for ongoing treatment
with an anticholinergic medicine, e.g. diagnosis of
urinary frequency, urgency or urge incontinence and
symptom improvement when taking the anticholinergic
medicine OR
- A record of a discussion with the patient about reducing
the dose or stopping the anticholinergic medicine
completely, or switching to an alternative medicine with
lower anticholinergic activity (if available)
Data analysis
Use the sheet provided to record your data. Aim to carry out
an assessment of anticholinergic burden for as many eligible
patients as possible.
Clinical audits can be an important tool to identify where gaps exist between expected and actual performance. Once completed, they can provide ideas on how to change practice and improve patient outcomes. General practitioners are encouraged to discuss the suitability and relevance of their proposed audit with their practice or peer group prior to commencement to ensure the relevance of the audit. Outcomes of the audit should also be discussed with the practice or peer group; this may be recorded as a learning activity reflection if suitable.
The Plan, Do, Study, Act (PDSA) model is recommended by the Royal New Zealand College of General Practitioners (RNZCGP) as a framework for assessing whether a clinical audit is relevant to your practice. This model has been widely used in healthcare settings since 2000. It consists of two parts, the framework and the PDSA cycle itself, as shown in Figure 1.
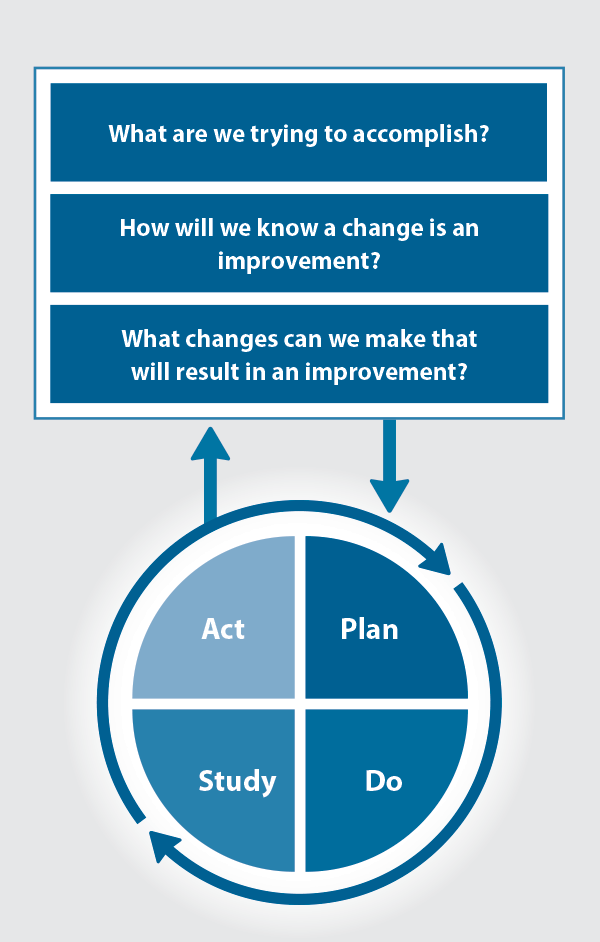
Figure 1. The PDSA model for improvement.
Source: Plan, Do, Study, Act (PDSA) cycles and the model for improvement
1. The framework
This consists of three questions that help define the “what” and “how” of an improvement project (in this case an audit).
The questions are:
- "What are we trying to accomplish?" – the aim
- "How will we know that a change is an improvement?" – what measures of success will be used?
- "What changes can we make that will result in improvement?" – the concept to be tested
2. The PDSA cycle
This is often referred to as the “engine” for creating, testing and carrying out the proposed changes. More than one cycle is usually required; each one is intended to be short, rapid and frequent, with the results used to inform and refine the next. This allows an ongoing process of continuous learning and improvement.
Each PDSA cycle includes four stages:
- Plan – decide what the change to be tested is and how this will be done
- Do – carry out the plan and collect the data
- Study – analyse the data, assess the impact of the change and reflect on what was learned
- Act – plan the next cycle or implement the changes from your plan

Claiming credits for Te Whanake CPD programme requirements
Practice or clinical audits are useful tools for improving clinical practice and credits can be claimed towards the Patient Outcomes (Improving Patient Care and Health Outcomes) learning category of the Te Whanake CPD programme, on a two credit per learning hour basis. A minimum of 12 credits is required in the Patient Outcomes category over a triennium (three years).
Any data driven activity that assesses the outcomes and quality of general practice work can be used to gain credits in the Patient Outcomes learning category. Under the refreshed Te Whanake CPD programme, audits are not compulsory and the RNZCGP also no longer requires that clinical audits are approved prior to use. The college recommends the PDSA format for developing and checking the relevance of a clinical audit.
To claim points go to the RNZCGP website: www.rnzcgp.org.nz
If a clinical audit is completed as part of Te Whanake requirements, the RNZCGP continues to encourage that evidence of participation in the audit be attached to your recorded activity. Evidence can include:
- A summary of the data collected
- An Audit of Medical Practice (CQI) Activity summary sheet (Appendix 1 in this audit or available on the
RNZCGP website).
N.B. Audits can also be completed by other health professionals working in primary care (particularly prescribers), if relevant. Check with your accrediting authority as to documentation requirements.





