Published: July, 2024 | Review date: July, 2027
This audit identifies patients who are prescribed warfarin and documents whether there is accurate and complete information recorded in their clinical notes to guide safe treatment.
Warfarin is frequently associated with adverse effects, as well as numerous medicine and food interactions. Since the introduction of direct oral anticoagulants (DOACs) in New Zealand, rates of warfarin prescribing have substantially decreased.1 DOACs are generally associated with superior clinical efficacy/safety, and have a number of practical advantages compared with warfarin, e.g. more predictable pharmacokinetic/pharmacodynamic properties (no INR monitoring required), fewer medicine and food interactions and a more rapid onset of action.2
However, there are some situations in which DOACs are contraindicated (e.g. mechanical heart valves) or there is insufficient evidence to support their use (e.g. moderate-to-severe mitral stenosis, severe liver or renal dysfunction), and warfarin should be used instead.2 Other reasons for considering warfarin include patients with a significant history of gastrointestinal disease or co-morbid antiphospholipid syndrome (rare) or patients who develop thrombosis while taking a DOAC.
For further information on anticoagulant selection and warfarin use, see: bpac.org.nz/2023/anticoagulants.aspx
Recommendations for safe warfarin use
If a decision is made to prescribe warfarin, it is essential that steps are taken to improve the safety of treatment. This includes:3
 Patient education – including information about bleeding risk, dietary considerations, medicines and ongoing/regular blood tests. All patients should be fully informed of these details prior to initiating anticoagulant treatment and reminded regularly during treatment. For patient education examples, see Appendix 1 of “Guideline for Warfarin Management in the Community”, available at: www.health.qld.gov.au/clinical-practice/guidelines-procedures/medicines/safety
Patient education – including information about bleeding risk, dietary considerations, medicines and ongoing/regular blood tests. All patients should be fully informed of these details prior to initiating anticoagulant treatment and reminded regularly during treatment. For patient education examples, see Appendix 1 of “Guideline for Warfarin Management in the Community”, available at: www.health.qld.gov.au/clinical-practice/guidelines-procedures/medicines/safety Accurate and complete recording of patient information – any prescriber should be able to easily access key information about the patient’s management. This includes that the patient is prescribed warfarin, the condition it is prescribed for, the brand of warfarin (there may be differences in bioavailability; brands are not interchangeable), the planned duration of treatment, the target INR range, when INR testing last occurred and when it should next occur. The latest INR result and current warfarin dose should also be clearly stated in the patient’s notes. The duration of warfarin treatment will vary depending on the indication; warfarin is typically taken for up to six month following deep vein thrombosis, whereas patients with atrial fibrillation and an elevated stroke risk usually require it lifelong.
Accurate and complete recording of patient information – any prescriber should be able to easily access key information about the patient’s management. This includes that the patient is prescribed warfarin, the condition it is prescribed for, the brand of warfarin (there may be differences in bioavailability; brands are not interchangeable), the planned duration of treatment, the target INR range, when INR testing last occurred and when it should next occur. The latest INR result and current warfarin dose should also be clearly stated in the patient’s notes. The duration of warfarin treatment will vary depending on the indication; warfarin is typically taken for up to six month following deep vein thrombosis, whereas patients with atrial fibrillation and an elevated stroke risk usually require it lifelong.- This information should be immediately obvious to anyone accessing the notes, and can be done in several ways in most practice management systems. For example, in Medtech a “screening” entry can be used to make the information easier to find rather than identifying it across multiple consultation notes, or medicines can be linked to the specific long-term classification that they are required for.
 Safe prescribing – all necessary information should be available to the pharmacist (on the prescription) and to the patient (on the medicine label). A prescription for warfarin should include:
Safe prescribing – all necessary information should be available to the pharmacist (on the prescription) and to the patient (on the medicine label). A prescription for warfarin should include:
- Relevant information from the patient’s clinical records noted above (e.g. the specific warfarin brand, dose, INR monitoring requirements). Also identify the clinician or service responsible for INR monitoring and warfarin dosing.
- Labelling that highlights the importance of ongoing INR monitoring, e.g. “Take the dose instructed by your doctor or nurse. You need regular INR tests to make sure this dose is safe for you”, instead of labels such as “PRN” or “as instructed”.
To minimise the potential for error and to ensure safe and effective anticoagulation, a systematic, practice-wide approach to warfarin treatment and maintenance of INR target levels is recommended. Common protocols should cover:
- How to initiate warfarin for patients in primary care, i.e. indications and recommended INR range, starting doses, who will follow up the patient and their warfarin treatment. For most people, e.g. those with atrial fibrillation, the recommended target INR range is 2.0 – 3.0. Some people with mechanical heart valves or haemodynamically significant valvular disease require a higher INR, e.g. within the range of 2.5 – 3.5.
- How to monitor warfarin treatment, being aware of significant medicine interactions, modifying warfarin dosage and frequency of INR testing
- A standardised method of recording clinical information for patients taking warfarin
- Access to vitamin K (phytomenadione) for reversal of anticoagulation if required – this may be stocked at the practice (available on PSO), or arrangements put in place to facilitate rapid access
References:
- Harper P, Chang A, Stephens M. The changing use of anticoagulants in New Zealand. N Z Med J 2022;135:35–43.
- Chen A, Stecker E, A. Warden B. Direct oral anticoagulant use: a practical guide to common clinical challenges. JAHA 2020;9:e017559. doi:10.1161/JAHA.120.017559
- Queensland Health. Guidelines for warfarin management in the community. 2024. Available from: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/medicines/safety (Accessed Jul, 2024).
Summary
This audit identifies patients in the practice currently prescribed warfarin to assess whether there is accurate and complete information recorded in their clinical notes to guide safe treatment. If all necessary information regarding warfarin treatment and INR testing requirements is not readily available in the patient’s clinical record or notes, they should be flagged for review.
Recommended audit standards
Given the level of risk associated with warfarin and the high number of associated adverse effects and medicine interactions reported each year in New Zealand, the standard for this audit should be high. A recommended standard for the first cycle would be for 90% of patients to have all the required information recorded in their notes. In addition, there should ideally be an improvement in the achieved percentage between the first and second audit cycles.
Eligible patients
All patients within the practice currently prescribed warfarin are eligible for this audit.
Identifying patients
You will need to have a system in place that allows you to identify eligible patients and audit their clinical notes. Many practices will be able to do this by running a ‘query’ through their PMS to find all patients currently taking warfarin.
Sample size
The number of eligible patients will vary according to your practice demographic. If you identify a large number of patients, take a random sample of 30 patients whose notes you will audit (the first 30 results returned is sufficiently random for the purposes of this audit).
Criteria for a positive outcome
For a positive audit result, the clinical record or notes of patients taking warfarin should contain all of the following information:
- The condition for which they are taking warfarin
- Their target INR range
- When warfarin was initiated
- The brand of warfarin
- The current warfarin dose
- Planned duration of treatment
- The date of their most recent INR test
- The result from their most recent INR test
- When their next INR test should be requested
Data analysis
Use the sheet provided to record your data. A positive result is any patient who has a tick in each of the “patient record” columns (A – I). The percentage achievement can be calculated by dividing the number of patients with a positive result by the total number of patients audited.
Clinical audits can be an important tool to identify where gaps exist between expected and actual performance. Once completed, they can provide ideas on how to change practice and improve patient outcomes. General practitioners are encouraged to discuss the suitability and relevance of their proposed audit with their practice or peer group prior to commencement to ensure the relevance of the audit. Outcomes of the audit should also be discussed with the practice or peer group; this may be recorded as a learning activity reflection if suitable.
The Plan, Do, Study, Act (PDSA) model is recommended by the Royal New Zealand College of General Practitioners (RNZCGP) as a framework for assessing whether a clinical audit is relevant to your practice. This model has been widely used in healthcare settings since 2000. It consists of two parts, the framework and the PDSA cycle itself, as shown in Figure 1.
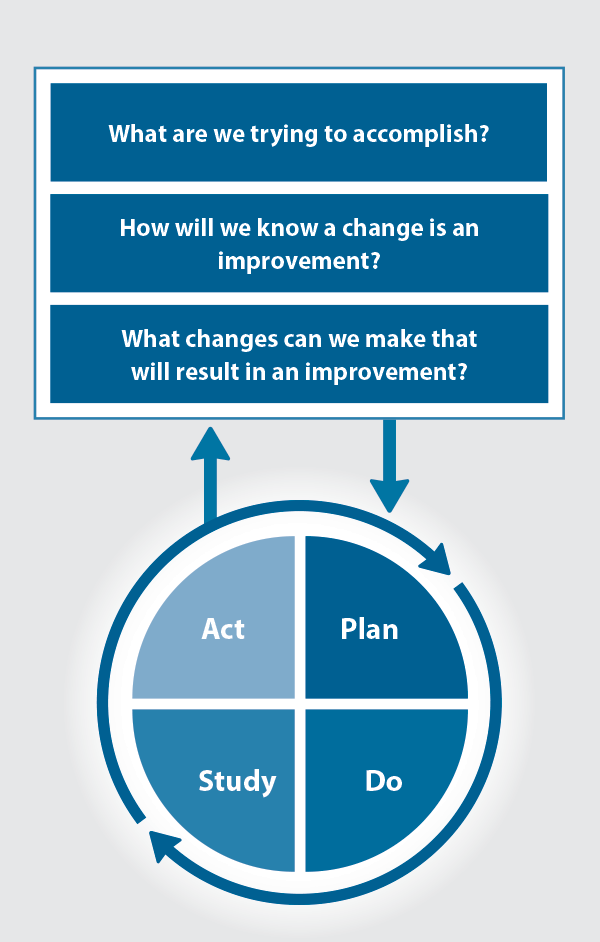
Figure 1. The PDSA model for improvement.
Source: Plan, Do, Study, Act (PDSA) cycles and the model for improvement
1. The framework
This consists of three questions that help define the “what” and “how” of an improvement project (in this case an audit).
The questions are:
- "What are we trying to accomplish?" – the aim
- "How will we know that a change is an improvement?" – what measures of success will be used?
- "What changes can we make that will result in improvement?" – the concept to be tested
2. The PDSA cycle
This is often referred to as the “engine” for creating, testing and carrying out the proposed changes. More than one cycle is usually required; each one is intended to be short, rapid and frequent, with the results used to inform and refine the next. This allows an ongoing process of continuous learning and improvement.
Each PDSA cycle includes four stages:
- Plan – decide what the change to be tested is and how this will be done
- Do – carry out the plan and collect the data
- Study – analyse the data, assess the impact of the change and reflect on what was learned
- Act – plan the next cycle or implement the changes from your plan

Claiming credits for Te Whanake CPD programme requirements
Practice or clinical audits are useful tools for improving clinical practice and credits can be claimed towards the Patient Outcomes (Improving Patient Care and Health Outcomes) learning category of the Te Whanake CPD programme, on a two credit per learning hour basis. A minimum of 12 credits is required in the Patient Outcomes category over a triennium (three years).
Any data driven activity that assesses the outcomes and quality of general practice work can be used to gain credits in the Patient Outcomes learning category. Under the refreshed Te Whanake CPD programme, audits are not compulsory and the RNZCGP also no longer requires that clinical audits are approved prior to use. The college recommends the PDSA format for developing and checking the relevance of a clinical audit.
To claim points go to the RNZCGP website: www.rnzcgp.org.nz
If a clinical audit is completed as part of Te Whanake requirements, the RNZCGP continues to encourage that evidence of participation in the audit be attached to your recorded activity. Evidence can include:
- A summary of the data collected
- An Audit of Medical Practice (CQI) Activity summary sheet (Appendix 1 in this audit or available on the
RNZCGP website).
N.B. Audits can also be completed by other health professionals working in primary care (particularly prescribers), if relevant. Check with your accrediting authority as to documentation requirements.





