Published: 15th November 2023 | Updated: 2nd April 2025
What's changed?
2nd April 2025
Update to Table 1, added BERIPLEX NZ to available VKA reversal agents
11th December 2023 Clarification around dosing of rivaroxaban in patients with renal impairment
If you would like to know what changes were made when the article was updated please contact us
Oral anticoagulants are used across a number of indications involving increased thrombosis risk. This article considers the situation where an oral anticoagulant is already indicated, and decisions are being made about risks versus benefits, and selecting the most appropriate option. For further information on managing atrial fibrillation, including guidance on how to decide if an oral anticoagulant should be used (e.g. with CHA2DS2-VASc), see: https://bpac.org.nz/2017/af.aspx
Key practice points:
- Increased bleeding risk is not an absolute contraindication to prescribing oral anticoagulants when they are indicated. Consider whether there are any modifiable risk factors present that can be managed before initiation, e.g. use of non-steroidal anti-inflammatory drugs, high alcohol intake, uncontrolled hypertension.
- Choose the most appropriate oral anticoagulant based on patient characteristics and co-morbidities. In most situations, direct oral anticoagulants (DOACs – including dabigatran and rivaroxaban) are favoured over warfarin in patients without contraindications, e.g. DOACs are superior for reducing the risk of stroke, all-cause mortality and intracranial bleeding in people with atrial fibrillation.
- DOACs also have a number of practical advantages compared with warfarin, e.g. more predictable pharmacokinetic and pharmacodynamic properties, no INR monitoring requirements, significantly fewer medicine and food interactions and more rapid onset of action
- Warfarin is preferred when there is insufficient evidence to support the use of a DOAC; this includes patients with moderate-to-severe mitral stenosis, severe liver or renal dysfunction, e.g. CrCl < 15 mL/min. N.B. If CrCl ≥ 15 mL/min DOAC use is possible with dose reduction (e.g. rivaroxaban) but if steadily declining then consider warfarin. Dabigatran should not be used if CrCl < 30 mL/min.
- Other situations where warfarin may be preferred include patients with a significant history of gastrointestinal disease or co-morbid antiphospholipid syndrome (rare) or patients who develop thrombosis while taking a DOAC (control can be more closely monitored through regular INR measurements)
- DOACs are contraindicated in patients with mechanical heart valves
- In patients where bleeding risk remains a concern, selecting an option with a reversal agent available is preferable, e.g. dabigatran or warfarin
- Oral anticoagulation should be avoided during pregnancy (low molecular weight heparins [LMWH] are preferred)
- Monitoring requirements differ according to the oral anticoagulant selected:
- Warfarin use requires ongoing and regular coagulation monitoring, i.e. INR testing, with corresponding dose adjustments
- DOAC use requires at least annual renal function testing; more regular review may be required for patients with co-morbidities or risk factors associated with CKD, e.g. Māori or Pacific ethnicity, diabetes or hypertension
- As co-morbidities emerge over time or new medicines are initiated, the suitability of the patient’s current anticoagulant should be reassessed. Regardless of the option selected, ongoing management should always consider modifiable risk factors for bleeding, treatment adherence and monitoring for adverse effects.
Anticoagulants function by interfering with coagulation pathways in the blood.1 This process reduces the probability of clot formation but predisposes patients to bleeding complications.1 As such, the decision to initiate oral anticoagulant treatment when indicated depends on determining that the risk of thrombosis is of greater clinical concern than the risk of bleeding.
Increased bleeding risk is not, however, an absolute contraindication to anticoagulation treatment. The first step after deciding that an oral anticoagulant is needed is to manage any modifiable risk factors for bleeding, e.g. uncontrolled hypertension, alcohol use greater than eight standard drinks per week.2 For certain indications, scoring tools may be useful in identifying modifiable bleeding risk factors, e.g. the HAS-BLED prediction tool in patients with atrial fibrillation (AF).2 In particular, consider whether the patient is taking any medicines that might increase their risk of bleeding, e.g. over-the-counter (OTC) non-steroidal anti-inflammatory drugs (NSAIDs). This should also include checking for medicines initiated in secondary care. For example, antiplatelets (e.g. aspirin, clopidogrel or ticagrelor) may be prescribed following admission for an acute coronary syndrome or rivaroxaban may be initiated after surgery.
Laboratory testing before treatment is initiated
If oral anticoagulation is required, laboratory investigations should be performed to assess for contraindications or risk factors before initiating treatment:2, 3
- Full blood count – platelets to exclude thrombocytopenia, haemoglobin to assess for anaemia
- Coagulation screen (varies depending on the laboratory, e.g. APTT, INR). N.B. Many primary care clinicians may not currently request a coagulation screen before starting a patient on an anticoagulant, but this is regarded as best practice to rule out pre-existing clotting disorders and to establish a baseline (if warfarin is ultimately used).
- Creatinine and electrolytes – to assess renal function, which may influence medicine selection and initial dosing (see: “Assess renal function before making anticoagulant selection”). This also provides a baseline for ongoing monitoring. Chronic kidney disease (CKD) is associated with an increased risk of bleeding in patients taking anticoagulants, particularly dabigatran.2, 4
- Liver function tests – severe liver dysfunction may influence oral anticoagulant clearance, thereby increasing the degree and/or duration of anticoagulation effect
For decades, warfarin was the only available oral anticoagulant in New Zealand. However, the introduction of direct oral anticoagulants (DOACs) has since provided alternative options, including dabigatran (fully funded since July, 2011), rivaroxaban (fully funded since August, 2018) and apixaban (not funded). Table 1 provides an overview of indications and factors to consider when discussing the advantages and disadvantages of DOACs and warfarin with patients.
For example, patients with:
- Multiple bleeding risk factors – consider choosing an option with a reversal agent available (in New Zealand), i.e. dabigatran or warfarin
- A significant history of dyspepsia, GORD, oesophagitis – consider avoiding dabigatran as use is associated with an increased risk of dyspepsia (see: “Managing dyspepsia associated with dabigatran use”)
- Potential adherence issues – consider an option with once daily dosing, e.g. rivaroxaban
- CKD or declining renal function – select an option that undergoes lower direct renal clearance, e.g. rivaroxaban, apixaban, warfarin (see: “Assess renal function before making anticoagulant selection”)
- Clinically significant valvular disease, e.g. moderate-to-severe mitral stenosis – select warfarin due to inadequate DOAC safety data (see: “Situations where warfarin use is recommended”)
- Mechanical heart valves – select warfarin as DOACs are contraindicated
Table 1. Properties of oral anticoagulants used in primary care. Adapted from Toma et al, 2022.1, 3, 5, 6
|
DOAC |
VKA |
Medicine |
Dabigatran |
Rivaroxaban |
Apixaban |
Warfarin |
Target |
Thrombin
(factor IIa) |
Factor Xa |
Vitamin K-dependent clotting (or coagulation) factors |
Half-life |
12 – 14 hours |
9 – 13 hours |
8 – 11 hours |
20 – 60 hours |
Onset of action |
2 hours |
2.5 – 4 hours |
3 hours |
Days |
Key monitoring requirements |
Renal function monitoring
(at least annually; more frequent if impaired at baseline) |
INR monitoring
(regular and ongoing) |
Funding status |
Fully funded |
Not funded |
Fully funded |
Tablet strengths available* |
75 mg
110 mg
150 mg |
10 mg
15 mg
20 mg |
2.5 mg
5 mg |
1 mg
2 mg
3 mg
5 mg |
Usual dosing frequency |
Twice day
(N.B. Once daily when used for VTE prophylaxis following surgery) |
Once daily |
Twice day |
Once daily |
Reversal agent |
Available
(idarucizumab) |
Unavailable in New Zealand
(options are available overseas) |
Available
(vitamin K, Prothrombinex-VF, BERIPLEX NZ) |
Key interactions |
- P-gp inhibitors† (increases DOAC concentration)
- P-gp inducers** (significantly decrease DOAC concentration)
- Antacids
|
- Strong CYP3A4 inhibitor + combined P-gp inhibitor, e.g. carbamazepine, rifampin, St John’s wort (significantly increase DOAC concentration)
- Moderate CYP3A4 inhibitor + combined P-gp inhibitor, e.g. clarithromycin, diltiazem (moderately increase DOAC concentration)
- Strong CYP3A4 inducer, e.g. phenytoin (significantly decrease DOAC concentration)
- P-gp inducers** (significantly decrease DOAC concentration)
|
- Numerous medicine interactions, e.g. erythromycin and other antibiotics, antifungals, amiodarone, NSAIDs; see NZF interaction checker for a comprehensive list
- Food interactions; particularly vitamin K rich foods, e.g. avocados, broccoli, leafy green vegetables such as spinach
|
Indications
(see NZF for indication-specific dosing regimens) |
All DOACs:
- Prevention of stroke and systemic embolism in non-valvular atrial fibrillation
- Treatment of deep-vein thrombosis and pulmonary embolism
- Prevention of recurrent deep-vein thrombosis or pulmonary embolism
- Prevention of venous thromboembolism following major joint surgery, e.g. hip, knee
|
Warfarin:
- Prevention and treatment of venous thrombosis and pulmonary embolism
- Prevention of stroke following myocardial infarction in patients with increased embolic risk
- Prevention of thromboembolism in patients with atrial fibrillation
- Prevention of thromboembolism in patients with prosthetic heart valves
|
Additional indication for rivaroxaban only:
Prophylaxis of cardiovascular events in patients with coronary artery disease or peripheral artery disease (in combination with aspirin) |
Contraindications‡ or insufficient evidence to support use in primary care
(see NZF for cautions)
N.B. See Figure 1 for further information on use in patients with renal dysfunction |
All oral anticoagulants:
- Active serious bleeding
- Certain bleeding-associated co-morbidities, e.g. severe thrombocytopenia or severe anaemia
- History of recent high-risk bleeding event, e.g. intracranial haemorrhage (N.B. Patients with recent intracranial haemorrhage may still benefit from oral anticoagulant use, however, these decisions should be made in secondary care following neuroimaging)
- Pregnancy (see: “Anticoagulation during pregnancy”)
|
All DOACs:
- Mechanical heart valves
- Moderate-to-severe mitral stenosis
- Severe liver disease
|
Warfarin:
Usually avoided in primary care for patients with severe renal impairment but may be considered under secondary care guidance, if required |
Dabigatran:
CrCl < 30 mL/min |
Rivaroxaban:
CrCl < 15 mL/min |
Apixaban:
CrCl < 25 mL/min (N.B. Use may still be considered under secondary care guidance if CrCl 15 – 24 mL/min) |
* As of November, 2023, two different warfarin brands are available in New Zealand. Coumadin is available in 1 mg, 2 mg and 5 mg strengths, and Marevan is available in 1 mg, 3 mg and 5 mg strengths. Avoid mixing brands to achieve appropriate dosing; they are not considered bioequivalent or interchangeable at any given tablet strength.
† Examples of P-gp inhibitors include amiodarone, clarithromycin, cyclosporine, erythromycin, ivacaftor, ketoconazole, nifedipine, ticagrelor, tolvaptan, verapamil. Interaction increases DOAC concentration or effect.
** Examples of P-gp inducers include carbamazepine, phenytoin, rifampicin, St John’s wort. Interaction significantly decreases DOAC concentration or effect.
‡ The distinction between relative and absolute contraindications for oral anticoagulant prescribing can be challenging as they are often context-specific and there are differing perspectives in the literature. The criteria listed here broadly encompass groups for whom oral anticoagulant initiation should be avoided in primary care; if there is any uncertainly, consult with secondary care.
CrCl = creatinine clearance; DOAC = direct oral anticoagulant; INR = international normalised ratio; P-gp = P-glycoprotein; VKA = vitamin K antagonist; VTE = venous thromboembolism
DOACs are generally preferred over warfarin unless contraindicated
In most patients with AF, DOACs are superior for reducing the risk of stroke and all-cause mortality.2, 7 They also reduce the risk of intracranial bleeding and have a comparable risk of major bleeding versus warfarin.2, 7 An analysis of more than 70,000 patients with AF demonstrated that standard dose DOACs reduced relative stroke or systemic embolism risk by 19% and intracranial haemorrhage risk by 55% compared with warfarin.7 Rates of major bleeding are also lower in patients with venous thromboembolism (VTE) treated using DOACs compared with warfarin.8 From a practical perspective, DOACs may be more convenient to use as they have more predictable pharmacokinetic and pharmacodynamic properties (no anticoagulation/INR monitoring required), significantly fewer medicine and food interactions and a more rapid onset of action compared with warfarin (Table 1).5, 9
Deciding between DOACs
All DOACs have similar clinical efficacy and are all suitable first-line options across approved indications.3, 10 The choice of DOAC should be made on a case-by-case basis depending on patient characteristics, but is usually between dabigatran and rivaroxaban because apixaban is not funded (as of November, 2023). While results from retrospective observational studies have suggested there are possible differences in clinical outcomes between the DOAC options,11 randomised controlled trials (RCTs) directly comparing them have not yet been completed, and any absolute differences in benefit or risk are likely small.
There is evidence that rates of menorrhagia are higher in females taking rivaroxaban compared with dabigatran.12 Therefore, selecting dabigatran may be a safer option in patients with a history of heavy menstrual bleeding.12 In addition, some evidence from registry-derived data suggests that rivaroxaban slightly increases the risk of gastrointestinal bleeding compared with other DOACs.13 While it has been proposed this is related to higher peak plasma levels associated with once daily dosing, the potential association needs to be investigated further, and such dosing convenience will likely outweigh bleeding concerns in patients without multiple risk factors in this context.14
Rivaroxaban is often the preferred funded option for treating deep-vein thrombosis and pulmonary embolism for practical reasons because it can be initiated immediately without the need for prior parenteral anticoagulant treatment (e.g. low molecular weight heparins - LMWH) as is required before treatment with dabigatran.
N.B. In patients with cancer-associated VTE, only Xa inhibitors (e.g. rivaroxaban, apixaban) have been compared in RCTs to the conventional treatment option, LMWH.15 Xa inhibitors reduce the risk of recurrent VTE in this context compared with LMWH, but were associated with an increased bleeding risk.15
Why consider apixaban if it is not funded?
In initial trials investigating different DOACs for the treatment of AF and deep-vein thrombosis/pulmonary embolism, apixaban appeared to exhibit the best safety profile, having the lowest incidence of thrombosis along with the lowest risk of bleeding.1, 3 However, as data from more clinical trials has become available over time, this potential advantage over other DOACs has become less certain.
There is some evidence that apixaban may have greater efficacy and safety in patients with VTE versus other DOACs, but absolute benefits demonstrated in clinical trials have been very small.3, 16 For example, in a retrospective analysis of > 37,000 patients with VTE, apixaban reduced the absolute probability of VTE recurrence and gastrointestinal/intracranial bleeding within six months by 0.011 and 0.015, respectively, compared with rivaroxaban.16 Study authors suggested differences in pharmacokinetic parameters (e.g. smaller fluctuations in plasma concentrations due to twice daily dosing with apixaban) may account for differences between treatment groups.16 Further robust RCT data is needed to confirm whether any potential benefits warrant preferential use of unfunded apixaban in patients with VTE when other DOACs still provide a considerable protective effect.
Situations where warfarin use is recommended
Patients with mechanical heart valves or moderate-to-severe mitral stenosis should use warfarin
Warfarin should be selected in patients with mechanical heart valves or moderate-to-severe mitral stenosis resulting from clinically significant valvular disease. The Phase II RE-ALIGN study involving 252 patients with mechanical aortic or mitral valves was halted prematurely due to excessive stroke and bleeding risk in the dabigatran treatment group.17 As a result, dabigatran is contraindicated in people with mechanical valves, and because this patient group was excluded from subsequent DOAC trials, efficacy and safety is not established for rivaroxaban and apixaban.3 These pivotal DOAC trials also excluded patients with moderate-to-severe mitral stenosis, meaning there is insufficient evidence to support use.
More evidence needed to determine best option for patients with bioprosthetic valves
There is increasing interest in the possible use of DOACs in patients with bioprosthetic valves. A 2022 observational analysis of 8,089 patients with bioprosthetic valve replacement found that DOAC use was associated with similar mortality, lower bleeding, but higher stroke risk compared with warfarin users.18 While further robust RCT investigation is required to determine the best oral anticoagulant choice in patients with bioprosthetic heart valves, these results indicate that the reasons for selecting warfarin over DOACs may be less clear cut.
Situations where warfarin may be preferable to DOACs include patients with:
- Severe renal impairment/kidney failure – see: “Assess renal function before making anticoagulant selection”
- Severe liver impairment – all DOACs undergo hepatic transformation to some extent, and there is limited safety and efficacy data for their use in cases of severe liver impairment. Warfarin is the only recommended option in this context, however, more frequent INR monitoring is required and use may not be suitable in all cases.3
- Significant history of gastrointestinal disease – standard dose DOACs are associated with a higher risk of gastrointestinal bleeding compared with warfarin. The risk is comparable between these treatment options at lower doses.7 Altered gastrointestinal anatomy (e.g. following bariatric surgery) may influence DOAC absorption more than warfarin.19
- Antiphospholipid syndrome – there is limited evidence suggesting DOACs are less effective for preventing recurrent thrombosis in patients with antiphospholipid syndrome versus warfarin, particularly if they have a history of arterial events20
- Recurrent thrombosis while taking a DOAC – although there is no clear evidence that warfarin is a better option in this situation, there may be some advantages to changing to warfarin, e.g. control can be closely monitored through regular INR measurements (N.B. In some cases a higher INR range may be required). Management of these patients usually requires input from secondary care.
- Significant adverse effects experienced with DOAC treatment (some patients can tolerate warfarin but not DOACs)
Assess renal function before making anticoagulant selection
All DOACs undergo some degree of direct elimination via the kidneys (Figure 1).3 Impaired kidney function linked either to pathology or medicine interactions can result in DOAC accumulation and increase bleeding risk further.3 The majority of dabigatran clearance occurs via the kidneys (approximately 80%) and therefore baseline renal function should be a key consideration before prescribing this medicine, and use is contraindicated if the CrCl is < 30 mL/min.21 Both rivaroxaban and apixaban are eliminated to a lesser extent by the kidneys, and therefore can be used in patients with more significant impairment (see Figure 1 for thresholds). Rivaroxaban can be used in patients with a CrCl of 15 – 29 mL/min, however, dose adjustment is required from 20 mg to 15 mg, once daily.5 N.B. Dose adjustment is also required for rivaroxaban in patients with a CrCl 30 – 49 mL/min.5
Warfarin is eliminated via hepatic metabolism rather than direct kidney excretion, meaning it is sometimes used in patients with a CrCl < 15 mL/min (i.e. kidney failure) and has been used safely in patients on dialysis.22 However, robust data supporting its safety and efficacy in this context is lacking and so warfarin is not routinely initiated in primary care for these patients.3 In general, the decision to prescribe anticoagulation at all in patients with a CrCl < 15 mL/min is highly individualised, and should be made under secondary care guidance.23
Use the Cockcroft-Gault equation for patients with reduced renal function
The Cockcroft-Gault equation is used to calculate CrCl (in mL/min) and guide oral anticoagulant dosing in preference to the estimated glomerular filtration rate (eGFR in mL/min/1.73m2) supplied by the laboratory. This is because the Cockcroft-Gault equation was used in the original Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study that validated dabigatran use, and subsequent investigations regarding DOAC efficacy and safety have also used these calculations.24, 25 Significant variations can occur between estimates of renal function provided by Cockcroft-Gault and eGFR in people who are obese or very slim or in older people with reduced muscle mass.3
A creatinine clearance calculator is available from the NZF: nzf.org.nz/nzf/resource/Creatinine%20Clearance%20Calculator.htm
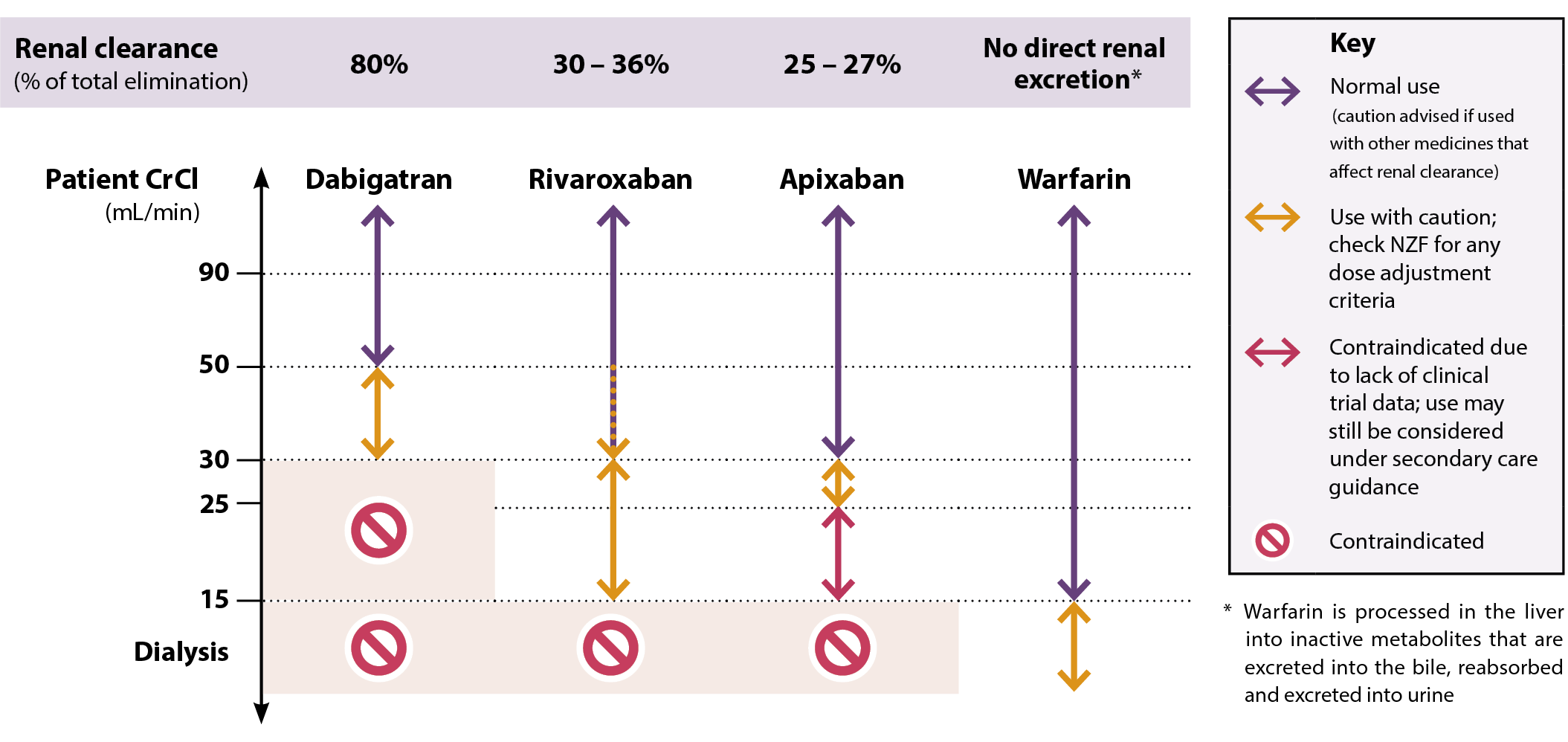
Figure 1. Oral anticoagulant selection in patients with renal impairment. Adapted from Mavrakanas et al, 2020.5, 21, 22
N.B. Rivaroxaban is usually appropriate in patients with a CrCl of 30 – 49 mL, but is prescribed with a lower dose (Table 2)
Weight may influence oral anticoagulant selection
There is concern that physiological differences attributable to extreme body weights can influence the clearance of DOACs, thereby increasing the risk of adverse effects.3 Optimal DOAC dosing strategies have not been established for morbidly obese patients, with guidelines generally only endorsing use in patients up to a BMI of 40 kg/m2 or body weight of 120 kg.3, 26 Beyond these thresholds, there is low quality evidence suggesting that rivaroxaban or apixaban may be preferable over dabigatran due to more favourable pharmacokinetic profiles.3, 26 Similarly, dabigatran should generally be avoided in patients with low body weight (e.g. < 50 kg), particularly those with renal impairment. Seek haematology advice if there are any concerns regarding DOAC selection or dosing in patients with extreme body weights.
A 2019 meta-analysis demonstrated that the risk of systemic embolism and major bleeding was reduced in patients with AF taking DOACs versus warfarin across underweight, normal weight and overweight categories.27 The associated risk of these outcomes was similar in obese patients between warfarin and DOAC treatment groups (i.e. non-inferior).27
Anticoagulation during pregnancy
Thrombophilia is a normal physiological change during pregnancy, increasing the risk of thromboembolism. For most people this does not result in any adverse outcomes, however, preventative anticoagulation treatment may be required for those with additional factors that increase this risk, e.g. VTE (personal or family history), AF, prosthetic heart valves, left ventricular dysfunction.28 This can pose a challenge because of possible fetal and maternal adverse effects associated with medicine use.
Anticoagulation decisions should usually be made under the guidance of an obstetrician, with use typically being reserved for patients at high risk.28 If required, anticoagulation with heparin (particularly LMWH such as enoxaparin) is generally considered the best approach during pregnancy for preventing and treating VTE or in the management of AF as they do not cross the placenta or cause fetal anticoagulation.2, 28 There is insufficient evidence to support the safety of DOAC use during pregnancy for any indication (major DOAC trials have excluded pregnant people). Warfarin is also generally avoided during pregnancy across all indications, but is sometimes considered at certain stages in patients at high risk under specialist guidance.2, 5 Warfarin should not be used during the first trimester due to possible teratogenic effects, as well as in the two to four weeks preceding delivery and within 48 hours post-partum to avoid fetal or maternal bleeding.2, 5 If anticoagulation is not suitable, electrical cardioversion can be performed safely at all stages of pregnancy, and should be considered in patients who are haemodynamically unstable due to AF or whenever the risk of ongoing AF is considered high to either the mother or the fetus.2
Patients already taking DOACs who are planning a pregnancy do not need to discontinue use prior to conception. Advise patients taking DOACs that if they become pregnant, they should book an appointment as soon as possible to discuss either temporarily discontinuing use or switching to LMWH treatment under close supervision.
Consider possible medicine interactions
Numerous medicines influence warfarin metabolism and therefore the presence of co-morbidities can significantly impact its suitability for specific patients (see: “Factors that can affect INR”).1 While comparatively fewer major interactions have been reported with DOACs, various medicines still increase bleeding risk or reduce clinical effect by altering absorption or elimination (Table 1).3 For example, dabigatran is a substrate for the P-glycoprotein (P-gp) efflux transporter located in the gut mucosa which regulates absorption; concurrent use of P-gp inducers (e.g. rifampicin) decreases plasma DOAC concentrations thereby increasing the risk of thrombosis, whereas P-gp inhibitors (e.g. amiodarone, erythromycin) increase plasma DOAC concentrations and increase bleeding risk.3
The risk of bleeding with oral anticoagulants is increased by concurrent use of antiplatelet medicines including aspirin, however, this may be necessary in some patients, e.g. following an acute coronary syndrome.3 Bleeding risk is also increased with concurrent use of other medicines that affect platelet function or coagulation such as NSAIDs, selective serotonin reuptake inhibitors (SSRIs), heparins.3
The NZF interactions checker provides details on medicines that interact with the various oral anticoagulant options, and the clinical significance of the interaction. Available from: www.nzf.org.nz
Recommended DOAC dosing varies based on the indication due to the different doses used in the original trials where safety and efficacy were established. For each DOAC there are at least two dose strengths (Table 2 provides examples for two main indications); the standard dose provides optimal antithrombotic activity, while dose reduction is reserved for patients in whom safety is a concern due to the presence of risk factors, e.g. bleeding risk, renal impairment, low body weight, frailty.5
Table 2. Recommended doses for DOACs in non-valvular AF and VTE. Adapted from Ballestri et al, 2023.29 For further dosing information, including DOAC doses for other indications (e.g. prevention of venous thromboembolism following major joint surgery) see the NZF.
|
Prevention of stroke and systemic embolism in non-valvular AF standard dose |
Dose reduction |
Prevention and treatment of DVT and pulmonary embolism standard dose* |
Dose reduction |
Dabigatran |
150 mg, twice daily |
110 mg, twice daily if:
- Aged 75 – 80 years with low thromboembolic risk and high bleeding risk; or
- Aged ≥ 80 years; or
- CrCl 30 – 49 mL/min
|
150 mg, twice daily, after at least five days of parenteral anticoagulant treatment. Continue for up to six months. |
Consider 110 mg, twice daily if:
- Aged 75 – 80 years with low thromboembolic risk and high bleeding risk; or
- Aged ≥ 80 years; or
- CrCl 30 – 49 mL/min
|
Rivaroxaban |
20 mg, once daily, with food |
15 mg, once daily if CrCl 15 – 49 mL/min |
Initially 15 mg, twice daily with food, for 21 days, then 20 mg, once daily, with food. After 6 – 12 months of treatment at 20 mg once daily, consider reducing dose to 10 mg daily, if risk of bleeding outweighs risk of recurrence. |
Consider a maintenance dose of 10 mg, once daily, after three to six months if CrCl is 15 – 49 mL/min and risk of bleeding outweighs risk of recurrent DVT or PE |
Apixaban
(not funded) |
5 mg, twice daily |
2.5 mg, twice daily if:
- CrCl 25 – 30 mL/min; or
- At least two of the following criteria:
- Aged ≥ 80 years
- Body weight ≤ 60 kg
- Serum creatinine ≥ 133 micromol/L
|
Initially 10 mg, twice daily, for seven days, then 5 mg, twice daily, then 2.5 mg, twice daily, after six months |
|
*Following initial management (between 5 – 21 days), the usual duration of primary treatment for VTE is three to six months.8, 30 After primary treatment is completed, a decision should be made to either discontinue use or continue secondary prevention of recurrent VTE, guided by risk factors and patient preference.8, 30 For recommendations regarding the duration of anticoagulant use in this context, see 2019 guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of VTE (available at: https://www.thanz.org.au/documents/item/414).
AF = atrial fibrillation; CrCl = creatinine clearance; DVT = deep-vein thrombosis; PE = pulmonary embolism; VTE = venous thromboembolism
Monitoring of renal function is required with DOACs
Renal function testing is recommended at least annually for patients taking DOACs due to their variable dependence on direct renal excretion.3 More frequent monitoring may be required for patients with co-morbidities or risk factors associated with CKD (e.g. Māori or Pacific ethnicity, diabetes or hypertension) or in situations where renal function may decline rapidly (e.g. dehydrating illness, initiation of a diuretic or hypovolaemia), particularly if they are taking dabigatran.3 Declining renal function or increasing age may necessitate a dose reduction, or switching to a different option for patients taking dabigatran (as it is predominantly excreted via the kidneys).3
Managing bleeding in patients taking a DOAC
The risk of bleeding in patients taking a DOAC is reduced by appropriate dosing and monitoring according to baseline risk assessment, e.g. HAS-BLED scoring for AF.
Patients with mild bleeding can be treated in primary care with mechanical compression and supportive measures.29, 31 Oral or topical tranexamic acid (15 mg/kg, three to four times daily; usual adult dose is 1 g), may be appropriate in some cases of major bleeding to increase clotting and prevent excessive blood loss.29, 31 Adequate fluid intake should be encouraged, particularly in the case of dabigatran to ensure continued excretion in the urine. Patients should immediately stop taking their DOAC as well as any other medicine with an anticoagulant effect (e.g. antiplatelets); delaying the next DOAC dose may be sufficient in some mild cases, however, discontinuation should be considered if clinically appropriate.31 Request full blood count, renal function and electrolytes (particularly calcium).31
Treatment of moderate-to-severe bleeding in a patient taking a DOAC generally requires consultation with a haematology service.31 For specific protocols, see: bpac.org.nz/2018/docs/dabigatran-rivaroxaban-bleeding-management.pdf
A reversal agent for dabigatran, idarucizumab, has been funded for use in New Zealand hospitals since September, 2016. Idarucizumab is able to rapidly inhibit dabigatran in patients who have uncontrolled bleeding or who need to undergo urgent surgery.29 A reversal agent for rivaroxaban and apixaban is available overseas (andexanet alfa),29 but not in New Zealand (outside of clinical trials).
Managing dyspepsia associated with dabigatran use
Dyspepsia is reported in 12 – 33% of patients taking dabigatran, depending on the dose and the length of treatment.32, 33 Taking dabigatran after a meal or concurrently with a proton pump inhibitor can limit or prevent dyspepsia, thereby the risk of rare complications such as contact ulceration of the oesophagus.34 Gastrointestinal symptoms are often transient and patients should be encouraged to persist with treatment. A switch to another DOAC may be appropriate if the patient finds the adverse effects intolerable.
Dabigatran is currently listed on Medsafe’s M2 Medicines Monitoring programme and prescribers are encouraged to report any adverse reactions to the Centre for Adverse Reactions Monitoring (CARM): pophealth.my.site.com/carmreportnz
Monitoring of warfarin treatment requires a systematic and practice-wide process to ensure consistent and optimal care. The target INR for most patients treated with warfarin is 2.5, with a clinically acceptable range of 2.0 – 3.0, or 2.5 – 3.5 for patients with mechanical heart valves.35 A “time in therapeutic range” of ≥ 70% is generally the goal of treatment,2 however, this may be difficult to achieve for many patients. Significant variations in INR (beyond what is expected) should be discussed with the patient to identify possible causes before adjusting the warfarin dose, e.g. change in diet, OTC medicine use.
In some cases, warfarin treatment may be monitored by a pharmacist as part of the Community Pharmacy-Based Anticoagulation Management Service (CPAMS).
Initiating warfarin
The regimen for initiating warfarin varies according to the indication and the patient’s clinical characteristics. Numerous protocols for warfarin initiation and monitoring exist; clinicians should select one that they prefer and use the same one consistently. A protocol from Queensland Health for initiating patients with AF on warfarin is given as an example in Table 3.35 Some regions in New Zealand will have their own protocols.
General principles include:35
- For patients with a lower risk of thrombosis, e.g. with AF, begin treatment with warfarin 3 mg, daily, with baseline and then weekly INR testing for the first two weeks, with subsequent dose adjustments as appropriate
- For patients with a higher risk of thrombosis, e.g. prophylaxis or treatment of deep-vein thrombosis, begin treatment with warfarin 5 mg, daily and concurrent subcutaneous LMWH (enoxaparin), with daily INR testing for the first five days (the higher frequency of INR testing is required due to the greater risk of bleeding and concurrent treatment with LMWH)
- Dose adjustments should be at least four days apart to allow for changes in steady state and older patients are more likely to have a slower response
- A system needs to be in place for the patient to record their current dose, any dose adjustment and their next required INR. General practices should also be able to record this information on their PMS. Prescribing 1 mg tablets may make it easier for the patient to adjust the dose, e.g. from 3 mg to 2 mg.
- A small number of patients may be resistant or hypersensitive to warfarin, e.g. due to genetic variations
- Once maintenance dosing begins, the frequency of INR testing and any dose adjustments are determined by the proximity of the patient’s INR to target and the stability of results. Patients with stable results only require testing every four to six weeks, or possibly every eight weeks. Testing INR more frequently than every three days is unnecessary for dosing but may occasionally be done for safety reasons.
Table 3. Example of a protocol for initiating and monitoring warfarin treatment in patients at low risk of thrombosis, e.g. with atrial fibrillation. Adapted from Queensland Health (2016)35 and Australasian Society of Thrombosis and Haemostasis (2013).36
Initiating warfarin for patients at low risk of thrombosis |
INR test |
INR level |
Daily warfarin dose (until next INR) |
Day 0 (baseline) |
≤ 1.4 |
3 mg |
Day 7 |
< 1.4 |
Confirm adherence, check medicine interactions and then increase to 6 mg and check INR on day 11 or 12 |
|
1.4 – 1.5 |
Increase to 5 mg |
|
1.6 – 1.8 |
Increase to 4 mg |
|
1.9 – 2.1 |
Maintain 3 mg |
|
2.2 -2.5 |
Reduce to 2.5 mg |
|
2.6 – 2.7 |
Reduce to 2 mg |
|
2.8 – 3.0 |
Omit one to two daily doses and reduce to 1 mg |
|
> 3 |
Stop warfarin and repeat INR in three to five days and restart at 1 mg |
Day 14 + |
Check INR and adjust dose if required (below)
Monitor INR every two weeks until INR is in range for two to three consecutive tests, then monitor every four to six weeks or possibly every eight weeks in patients who are stable |
Maintenance dosing of warfarin |
|
INR level |
Dose adjustment |
|
< 1.5 |
Increase weekly dose by 20% and re-check INR |
|
1.5 – 1.9 |
No adjustment – re-check INR in one week and if persistent increase weekly dose by 10% |
|
2 – 3 |
No adjustment |
|
3.1 – 3.9 |
No adjustment – re-check INR in one week and if persistent decrease weekly dose by 10 – 20% |
Managing high INR or bleeding |
Clinical scenario |
|
Recommended action |
No bleeding |
INR above therapeutic range but < 4.5 |
Lower or omit the next warfarin dose.
Resume treatment at a lower warfarin dose when INR approaches therapeutic range (N.B. If INR is < 10% above therapeutic range then dose reduction may not be necessary). |
|
INR 4.5 – 10 |
Stop warfarin and consider reasons for elevation. Vitamin K is not routinely required unless bleeding risk is high* as restabilising INRs can be difficult.
Check INR within 24 hours and resume warfarin once INR approaches therapeutic range. |
|
INR > 10 |
Stop warfarin and administer vitamin K either 3 – 5 mg orally† or 0.5 – 1 mg intravenously.
Check INR in 12 – 24 hours and continue monitoring every one to two days for the following week.
Stop warfarin and administer vitamin K either 3 – 5 mg orally† or 0.5 – 1 mg intravenously.
Check INR in 12 – 24 hours and continue monitoring every one to two days for the following week.
Resume warfarin at a lower dose once INR approaches therapeutic range. |
Spontaneous bleeding or injury |
Any INR with minor bleeding |
Omit the next dose of warfarin.
Repeat INR the next day and adjust to warfarin dose to maintain INR in the therapeutic range. If bleeding risk is high or INR > 4.5 consider administering vitamin K. |
|
INR ≥ 2 with clinically significant bleeding |
Send immediately to hospital and administer vitamin K if there will be a delay in transportation. |
|
INR ≥ 1.5 with life-threatening bleeding |
* A major bleed in the last four weeks or major surgery in the last two weeks, liver disease, concurrent antiplatelet treatment, thrombocytopenia with platelets < 50 × 109/L
† For oral administration of vitamin K, use the injection preparation orally
For a warfarin initiation protocol for high-risk patients, e.g. for DVT prophylaxis or treatment, see: Guidelines for warfarin management in the community. Queensland Health. Available from: www.health.qld.gov.au/__data/assets/pdf_file/0025/443806/warfarin-guidelines.pdf
For further information on managing high INR and bleeding with warfarin, see:
Factors that can affect INR
 Medicine interactions. Numerous medicines interact with warfarin, either by altering the INR (e.g. antibiotics) or by increasing the risk of bleeding (e.g. NSAIDs or SSRIs). Information on medicine interactions and their clinical significance can be checked in the New Zealand Formulary.5 If a medicine needs to be initiated that interacts with warfarin, check the patient’s INR two to three days later and continue to monitor every two to three days while treatment continues or until their INR is stable.35 Also consider:
Medicine interactions. Numerous medicines interact with warfarin, either by altering the INR (e.g. antibiotics) or by increasing the risk of bleeding (e.g. NSAIDs or SSRIs). Information on medicine interactions and their clinical significance can be checked in the New Zealand Formulary.5 If a medicine needs to be initiated that interacts with warfarin, check the patient’s INR two to three days later and continue to monitor every two to three days while treatment continues or until their INR is stable.35 Also consider:
- Complementary and alternative medicine use, including Rongoā rākau.35 Certain substances should be avoided (e.g. St John’s wort, dong quai, danshen, natto) whereas others may be acceptable if they are discussed and monitored (e.g. ginkgo biloba, garlic- or ginger-containing products).
- OTC medicines.35 Educate the patient to discuss any OTC medicine choices with their pharmacist first and check for possible warfarin interactions. Emphasise avoiding OTC NSAID use.
 Co-morbidities or acute illness. Encourage patients to report any changes in their health status as this can affect INR levels. For example, an adjustment in warfarin dosing may be required for patients who develop features associated with acute illness (e.g. diarrhoea, fever), heart failure, hyper- or hypothyroidism, declining renal function or chronic liver disease.35
Co-morbidities or acute illness. Encourage patients to report any changes in their health status as this can affect INR levels. For example, an adjustment in warfarin dosing may be required for patients who develop features associated with acute illness (e.g. diarrhoea, fever), heart failure, hyper- or hypothyroidism, declining renal function or chronic liver disease.35
 Significant dietary changes. Foods that contain high levels of vitamin K can affect INR, e.g. broccoli, Brussels sprouts, or green leafy vegetables such as spinach, cabbage and lettuce.35 Rather than avoiding these foods, a consistent and balanced diet is recommended for patients to maintain a stable INR. Drinking excessive alcohol or consuming large quantities of cranberry juice or cranberry-based products may cause an interaction with warfarin and increase the risk of bleeding.35
Significant dietary changes. Foods that contain high levels of vitamin K can affect INR, e.g. broccoli, Brussels sprouts, or green leafy vegetables such as spinach, cabbage and lettuce.35 Rather than avoiding these foods, a consistent and balanced diet is recommended for patients to maintain a stable INR. Drinking excessive alcohol or consuming large quantities of cranberry juice or cranberry-based products may cause an interaction with warfarin and increase the risk of bleeding.35
Assuming there are no contraindications, switching from warfarin to a DOAC is appropriate if a patient’s INR is not stable despite following optimal management recommendations.37 This includes patients:37
- With two INR values higher than five or one value higher than eight within the last six months
- With two INR values less than 1.5 within the last six months
- Spending less than 65% of the time in the therapeutic range
Adherence should always be considered in patients with unstable INRs before changing treatment.37 While there are no recommendations for switching to DOACs in patients currently taking warfarin that have stable INR measurements, the option to transition should still be discussed with the patient due to the potential long-term benefits (taking into account their time in therapeutic range).37
For an overview of recommendations for switching between oral anticoagulants, see Table 4. This includes recommendations for switching between DOACs, and from a DOAC to warfarin in cases where contraindications arise during treatment, e.g. declining renal function.
Table 4. Quick reference guide for switching between oral anticoagulants.24, 38–40
|
Switching to: |
Switching from: |
Warfarin |
Dabigatran |
Rivaroxaban |
Apixaban |
Warfarin |
– |
Stop warfarin, measure INR daily, initiate dabigatran when INR < 2.0 |
Stop warfarin, measure INR daily, initiate rivaroxaban when INR is ≤ 3.0 if taking for stroke/systemic embolism prevention or when INR is ≤ 2.5 if taking for treatment or prevention of DVT and PE |
Stop warfarin, measure INR daily, initiate apixaban when INR < 2.0 |
Dabigatran |
If CrCl ≥ 50 mL/min, start warfarin three days before stopping dabigatran.
If CrCl 30 – 49 mL/min, start warfarin two days before stopping dabigatran. |
– |
Take first dose of rivaroxaban 12 hours after last dose of dabigatran |
Take first dose of apixaban 12 hours after last dose of dabigatran |
Rivaroxaban |
Initiate warfarin while still taking rivaroxaban, withdraw rivaroxaban when INR is ≥ 2.0.
Start warfarin at a standard dose and adjust dose based on INR after two days. |
Take first dose of dabigatran 24 hours after last dose of rivaroxaban |
– |
Take first dose of apixaban 24 hours after last dose of rivaroxaban |
Apixaban |
Initiate warfarin and continue taking apixaban for two days after the first dose. After two days of co-administration, obtain an INR prior to next scheduled dose of apixaban. Withdraw apixaban if INR is ≥ 2.0, otherwise continue co-administration with daily INR monitoring until target achieved. |
Take first dose of dabigatran 12 hours after last dose of apixaban |
Take first dose of rivaroxaban 12 hours after last dose of apixaban |
– |
Two free applications (“apps”) have been developed Dr Paul Harper, Clinical Haematologist, Te Whatu Ora MidCentral, to aid clinicians in managing patients taking dabigatran or rivaroxaban. The apps assist in selecting an appropriate dose for each indication based on the patient’s age and renal function. They also include relevant medicine information (tablet sizes, pharmacology, storage and advice about taking the medicine), specific dosing instructions and information about adverse effects, interactions and actions to take if a patient is bleeding.
Download the dabigatran or rivaroxaban apps for free from:
A New Zealand Blood Service app called “Reversing Warfarin” is also available:
Patient resources
Patient information sheets (including practical advice around dosing, and when to seek medical attention):
