In this article 
View / Download
pdf version of this article
Update: one fully subsidised brand for quetiapine, risperidone and olanzapine, BPJ62
| Key concepts |
- Use of atypical antipsychotics, such as quetiapine and olanzapine, is increasing and in many cases, they are being
prescribed “off-label” – this is a worrying trend due to their potential to cause harm
- Atypical antipsychotics should only be used for specific indications and used with caution, especially in elderly
people and young adults
|
- Atypical antipsychotics are indicated for the treatment of schizophrenia and related disorders and in some circumstances
to treat the behavioural and psychological symptoms associated with dementia (risperidone only)
- Antipsychotics are not a first-line treatment for anxiety and are not recommended for post-traumatic stress disorder
or insomnia
|
Prescribing of atypical antipsychotics is increasing
Antipsychotic medicines are classified as “typical” or “atypical”. Typical antipsychotics, also
known as traditional or first-generation antipsychotics, include haloperidol and chlorpromazine. Atypical antipsychotics,
also known as second generation antipsychotics, include quetiapine, risperidone and olanzapine. Both types of antipsychotics
act in a similar way by blocking receptors in the dopamine pathway, but atypical antipsychotics are less likely to cause
the extrapyramidal adverse effects associated with the older typical antipsychotics. However, atypical antipsychotics,
along with typical antipsychotics, are associated with serious adverse effects, such as diabetes mellitus, stroke and
cardiac death.1
Antipsychotics are indicated for the treatment of schizophrenia and related disorders and in some circumstances to treat
the behavioural and psychological symptoms associated with dementia (risperidone only). International experience shows
that antipsychotics are being increasingly used for off-label conditions (e.g. anxiety, insomnia).2,3
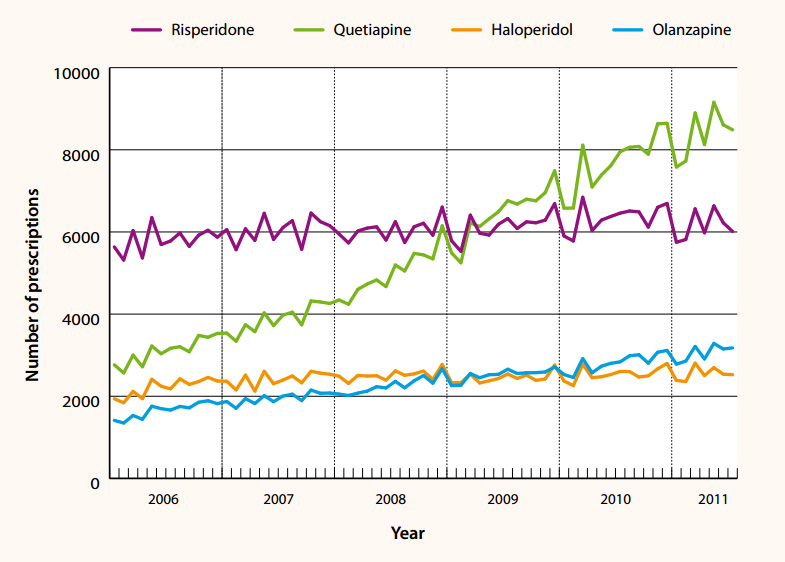
The use of atypical antipsychotics has been increasing in New Zealand (Figure 1) and it is likely that off-label use
is a factor in this increase. Special Authority prescribing restrictions have recently been removed from olanzapine (see
below). Risperidone (excluding depot injection and dissolving tablets) and quetiapine have been available without
restriction since 2009 and 2008 respectively.
Traditionally, General Practitioners have been responsible for writing repeat prescriptions for patients, once an atypical
antipsychotic has been initiated by a psychiatrist after confirmation of a diagnosis such as schizophrenia.
If a General Practitioner wishes to initiate treatment, then it is best to only prescribe for the recognised indications
and to discuss the treatment plan with a psychiatrist (or other relevant clinician) before prescribing. Be particularly
cautious when prescribing these medicines for elderly people, younger people and those at increased cardiovascular risk,
especially as a result of obesity, diabetes or high cholesterol.
Figure 1: Use of selected typical and atypical antipsychotics in
patients > 65 years by General Practitioners in New Zealand, 2006–2011 (dispensing data from the Pharmaceutical
Warehouse database).
Adverse effects of atypical antipsychotics
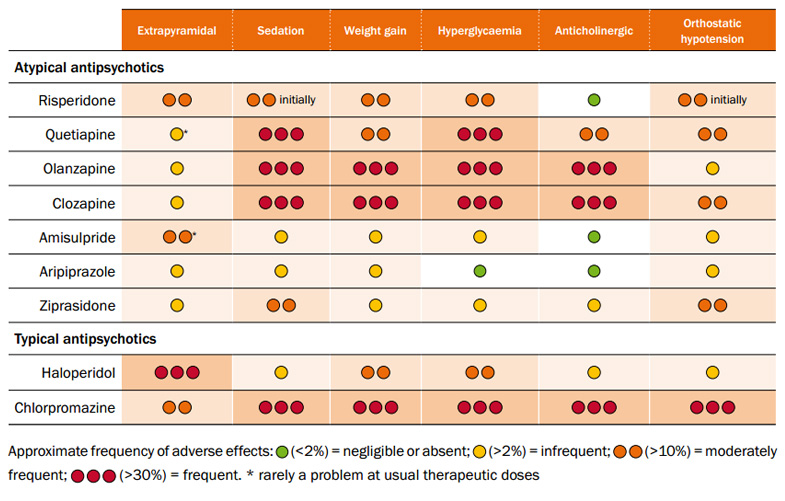
Most adverse effects are common to all antipsychotics, both typical and atypical, but occur to varying degrees for individual
medicines (Table 1).
Table 1 (click to enlarge): Relative frequency of common adverse effects of antipsychotics
(Psychotropic Expert Group, 2008).6
Common, dose related adverse effects include:4
- Sedation – especially with clozapine, olanzapine and quetiapine
- Anticholinergic effects such as dry mouth, constipation and blurred vision – especially with clozapine and olanzapine
- Dizziness and postural hypotension – especially with clozapine, risperidone and quetiapine
- As experience has grown, significant adverse effects associated with atypical antipsychotics have emerged, such as
the development of diabetes, dyslipidaemia, weight gain, metabolic syndrome, increased risk of stroke (particularly among
elderly people), elevated risk of sudden cardiac death, seizures and tardive dyskinesia.5 These adverse effects
are also associated with typical antipsychotics.
Metabolic adverse effects
Metabolic adverse effects associated with antipsychotics are of particular concern because of the increase in cardiovascular
morbidity and mortality.4 Olanzapine and clozapine are particularly associated with substantial weight gain,
dyslipidaemia and hyperglycaemia.4 People taking these medicines often report that they always feel hungry.
Weight gain can be substantial – a gain of 2 kg in two weeks should prompt a medicine review. All patients prescribed
antipsychotics should be given appropriate advice on diet and lifestyle interventions and monitored carefully for diabetes.7
 See: “Monitoring
for metabolic disorders in patients taking antipsychotic drugs”, BPJ 3 (Feb, 2007)
See: “Monitoring
for metabolic disorders in patients taking antipsychotic drugs”, BPJ 3 (Feb, 2007)
Extrapyramidal effects
Atypical antipsychotics are generally considered to cause fewer extrapyramidal adverse effects than typical antipsychotics.
A meta-analysis showed clozapine, olanzapine and risperidone to be significantly less commonly associated with extrapyramidal
symptoms than low potency typical antipsychotics (i.e. chlorpromazine 600 mg daily or equivalent).8 The majority
of studies have found no differences within the atypical group in terms of extrapyramidal effects.5
Tardive dyskinesia
Rates of new-onset tardive dyskinesia (orofacial and trunk movements) have been estimated at 3% with risperidone and
1% to 2% for other atypical antipsychotics.5 In comparison, tardive dyskinesia develops in around 20% of people
receiving typical antipsychotics.9 Tardive dyskinesia is of particular concern as it may not be evident immediately,
is often resistant to treatment, may be persistent and may worsen on treatment withdrawal.
Atypical antipsychotics for the treatment of schizophrenia and related disorders
Patients with schizophrenia should ideally be managed by a multidisciplinary team, including both primary and secondary
care.10 The early detection and prompt treatment of a first episode of schizophrenia is essential as this can
improve health outcomes and possibly even the progression of the illness.10
Typical and atypical antipsychotics are used in the treatment of schizophrenia. Both types of antipsychotics are associated
with adverse effects and there is also great variability in individual patient response. Therefore the same medicine cannot
be recommended for every patient. As a general principle, an antipsychotic is started at a low dose and carefully titrated
upwards to avoid or reduce the occurrence of adverse effects.6
Australasian guidelines recommend an atypical antipsychotic as first-line treatment for schizophrenia due to their lower
risk of extrapyramidal adverse effects compared to typical antipsychotics.10 However, more recently this recommendation
has been challenged due to the concern that metabolic adverse effects associated with atypical antipsychotics are more
problematic in the longer term.11
Clozapine is recommended in cases of treatment resistance, after the patient has trialled at least two other antipsychotics.10 This
medicine is not initiated in primary care, however, primary care clinicians have a role in monitoring for adverse effects
(see below).
Pharmacological treatments for schizophrenia should always be used in conjunction with comprehensive psychosocial interventions.10
Monitoring requirements for clozapine
Patients using clozapine require close monitoring as it can cause neutropenia, which may progress to a potentially
fatal agranulocytosis.
Blood tests (white cell count and absolute neutrophil count) are required:12
Patients who present with evidence of infection, e.g. sore throat, fever or flu-like symptoms require
an urgent complete blood count. Patients should also be advised to report any such symptoms.
- Ten days before commencing treatment
- Each week of the first 18 weeks of treatment
- Every four weeks during treatment
- Four weeks after discontinuation
- After discontinuation due to abnormal blood tests, until levels return to normal
Other adverse effects
Constipation is commonly associated with clozapine use and can be severe and even life-threatening. Co-prescription
of a laxative for patients taking clozapine is recommended.
Additional adverse effects of clozapine are similar to other antipsychotics, but antimuscarinic effects (e.g. dry mouth,
blurred vision, urinary retention), sedation and weight gain are often more pronounced.
The concomitant use of some medicines may increase the risk of adverse effects due to:
- A potential for bone marrow suppression, e.g. trimethoprim, co-trimoxazole, nitrofurantoin, sulphonamides, carbamazepine
- An increase in the plasma concentration of clozapine, e.g. erythromycin, ciprofloxacin
Clozapine is classified as a “hospital pharmacy” medicine which means it is only dispensed from a limited
number of authorised pharmacies. *>
 See “Clozapine: A reminder
about safe and effective use”. BPJ 14 (Jun, 2008).
See “Clozapine: A reminder
about safe and effective use”. BPJ 14 (Jun, 2008).
Atypical antipsychotics for the treatment of behavioural and psychological symptoms of dementia
Behavioural and psychological symptoms of dementia (BPSD) include; aggressiveness (verbal outburst, physical violence),
activity disturbance (agitation, wandering) and psychotic symptoms. Non-pharmacological treatment is recommended first-line,
but in some severe cases antipsychotics are used to manage BPSD.
Risperidone is the only atypical antipsychotic officially indicated for BPSD. Antipsychotics provide relatively few
clinical benefits for people with dementia and in some cases pose a serious risk of an adverse outcome. A recent review
predicts that for every 100 people with dementia given an antipsychotic only 20 will derive some clinical benefit and
there will be one extra death and one extra stroke.13
Atypical antipsychotics should be used with extreme caution in older people, due to an increased incidence of adverse
effects, and only used to treat severe symptoms. The decision to use an antipsychotic should be discussed with the patient,
family and caregivers. Initial doses should be reduced to half the adult dose or less, taking into account factors such
as the patient’s weight, co-morbidities, and concomitant medication. Treatment should also be reviewed regularly.14
Antipsychotic treatment is not effective for symptoms such as wandering, social withdrawal, shouting, pacing, touching,
cognitive defects and incontinence. Patients with these symptoms may respond to interventions such as improvements to
the environment.15
 See: “Antipsychotics
in people with dementia – an update and reminder”, BPJ 26 (Mar, 2010)
See: “Antipsychotics
in people with dementia – an update and reminder”, BPJ 26 (Mar, 2010)
Off-label uses of atypical antipsychotics: insomnia, anxiety and post-traumatic stress disorder
In a recent survey in Canterbury, 96% of psychiatrists reported that they prescribed antipsychotics for off-label uses,
with quetiapine being the most commonly used. The three most frequent indications for off-label prescribing of quetiapine
were; anxiety (89%), sedation (79%) and post-traumatic stress disorder (57%).16 Overall, it is estimated that
between 43% and 70% of atypical antipsychotics prescribed are used for off-label indications.16 It is generally
accepted that there is limited evidence to support the use of antipsychotics for off-label uses, but this practice is
now widespread and there is little guidance as to whether it needs to be reduced, or if patients are genuinely benefitting
from the use of these medicines for these indications.
Drug company marketing has played a strong role in the increase in use of antipsychotics in recent years. In the United
States, two drug manufacturers settled out of court, after being charged with illegally promoting the off-label use of
olanzapine and quetiapine.17,18 There are reports that quetiapine is increasingly emerging as a drug of misuse
in the United States. This trend has not yet been widely reported in New Zealand,19 but may appear over time.
There are some anecdotal reports that quetiapine is a drug of misuse in New Zealand prisons.
Off-label prescribing is legal in most countries, including New Zealand, therefore atypical antipsychotics can be prescribed
for indications such as anxiety. However, the prescriber needs to carefully weigh up the risks and benefits and consider
other appropriate medicines (or non-pharmacological options) first. The decision to prescribe should be discussed with
the patient and their family and carefully documented in the patient’s notes.
Anxiety
A selective serotonin re-uptake inhibitor (SSRI) is the first-line pharmacological treatment for generalised anxiety
disorder.20 Psychological treatments, such as cognitive behavioural therapy, are equally effective.21 Patients
should be treated for at least 12 weeks before assessing the efficacy of treatment with a SSRI. Treatment may need to
continue for six to 12 months after symptoms of anxiety have resolved.20
Benzodiazepines are sometimes trialled for the treatment of anxiety if SSRIs or psychological therapies have been ineffective.22 However,
there is significant concern with tolerance, dependence and misuse associated with this medicine class. Buspirone is funded
with Special Authority restriction, for use as an anxiolytic when other medicines are contraindicated or have failed.
Tricyclic antidepressants may also be considered in some cases.
There is some evidence that quetiapine may be effective in the treatment of generalised anxiety disorder.23,24 Due
to the adverse effects associated with quetiapine, it should only be considered for the short-term treatment of anxiety
if all other appropriate pharmacological or psychological treatments had been trialled and were ineffective.
 For further information see: “Generalised
anxiety disorder in adults”, BPJ 25 (Dec, 2009).
For further information see: “Generalised
anxiety disorder in adults”, BPJ 25 (Dec, 2009).
Post-traumatic stress disorder (PTSD)
PTSD is a psychiatric disorder which develops after exposure to a traumatic event. Three clusters of symptoms are typically
present – re-experiencing, avoidance and hyperarousal. Treatment for PTSD involves a combination of psychological therapies,
pharmacological treatment and social support. The first-line pharmacological treatment is an antidepressant, usually a
SSRI.
There is a lack of evidence to support the use of quetiapine (or other antipsychotics) for the treatment of PTSD.22
Insomnia
Insomnia is usually secondary to an underlying cause such as an illness or poor sleep environment. Investigation and
treatment of the underlying cause will usually resolve symptoms of insomnia.
The first-line treatment is “sleep hygiene” intervention, e.g. advice to avoid alcohol, nicotine and caffeine,
avoid stimuli before bedtime, avoid being in bed when not sleeping and learning relaxation techniques.
If this approach fails to resolve the insomnia, pharmacological treatment may be considered for short-term use. Zopiclone
or shorter-acting benzodiazepines (e.g. temazepam) are appropriate choices. Use the lowest effective dose for the shortest
possible time (less than four weeks and preferably five to ten days).25 Antidepressants are not recommended
for insomnia in the absence of depression or anxiety.26
Quetiapine is not recommended for the treatment of insomnia in the absence of psychiatric disorder, e.g. schizophrenia.
Quetiapine has been reported to increase total sleep time and sleep efficiency, however, adverse effects such as leg movements
and akathisia (restlessness) limit its effectiveness.27
 For further information see: “Managing
insomnia”, BPJ 14 (Jun, 2008).
For further information see: “Managing
insomnia”, BPJ 14 (Jun, 2008).
Olanzapine generic now available – weight gain and diabetes a concern
From 1 June 2011 two generic versions of olanzapine became available for prescription, without restriction – Olanzine
and Dr Reddy’s Olanzapine (tablet and dissolving forms). The Zyprexa brand of olanzapine is still available, but
is now subject to a significant part-charge. Some patients may need to be counselled through the brand change. Inform
patients that the orodispersible tablets taste different and may take longer to dissolve.
Olanzapine is approved for the treatment of schizophrenia and related psychoses and bipolar depression. Concern has
been expressed, now that olanzapine can be prescribed without a Special Authority approval, that it will be prescribed
for off-label indications in the same manner that has occurred for quetiapine. There are a number of conditions in which
olanzapine has been used, or is under investigation for use, including Alzheimer’s dementia, anorexia, autism,
Asperger’s disorder, insomnia, anxiety and agitation. As yet, there is no compelling evidence of its effectiveness
for these conditions.
When prescribing olanzapine the clinician should be particularly aware of the metabolic adverse effects including weight
gain, raised lipid levels, impaired glucose tolerance and new-onset diabetes.5 Pancreatitis is also reported
as a rare complication of olanzapine use.28
Table 2: Serious adverse effects of atypical antipsychotics that may be more troublesome
with olanzapine5,29,30,31
| Adverse effect |
Comment |
| Weight gain |
Weight gain in people using olanzapine can be 1 to 3 kg greater than that associated with other atypical antipsychotics
such as risperidone.
In long-term studies (at least 48 weeks) the mean weight gain was 5.6kg.
The proportion of patients with clinically important weight gain could be reasonably high as the number needed to
harm has been calculated as 4 for a ≥ 7% gain in body weight.
Teenagers (age 13–17 years) are more likely to gain weight and to gain more weight than adults.
Potential consequences of weight gain should be considered prior to starting olanzapine.
Monitor weight, waist circumference and BMI. If weight gain is more than 2 kg during the first two weeks then a
change of antipsychotic may be necessary. |
| Diabetes |
There is an increased risk of type 2 diabetes in people with schizophrenia.
Olanzapine has been associated with a greater risk of new-onset diabetes compared with the other atypical antipsychotics.
Use with caution in people with diabetes or other disorders of glucose regulation. Monitor HbA1c for signs of worsening
glucose control.
Avoid use in people with risk factors for diabetes e.g. obesity or family history. If used, monitor fasting glucose
levels before and periodically during treatment. |
| Serum lipids |
Olanzapine has been associated with increases in triglycerides, LDL cholesterol and total cholesterol and decreases
in HDL cholesterol.
Use with caution in patients with pre-existing abnormal lipid profile.
Monitor lipid levels during treatment. |
Acknowledgement
Thank you to Dr Erik Monasterio, Senior Clinical Lecturer, Christchurch School of Medicine, University
of Otago, Consultant in Forensic Psychiatry, Hillmorton Hospital and Andrew McKean, Acting Senior Pharmacist,
The Princess Margaret Hospital, Christchurch for expert guidance in developing this article.
References
- Ray W, Chung C, Murray K, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med
2009;360(3):225-35.
- Domino M, Swartz M. Who are the new users of antipsychotic medications? Psychiatr Serv 2008;59(5):507-14.
- Alexander G, Gallagher S, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States,
1995–2008. Pharmacoepidemiology and Drug Safety, 2011; 20: 177–84.
- Mackin P, Thomas S. Atypical antipsychotic drugs. BMJ 2011;342:650-4.
- McDonagh M, Peterson K, Carson S, et al. Drug class review: Atypical antipsychotic drugs. Update 3. Available from: http://derp.ohsu.edu/final/AAP_final_report_update_3_version%202_JUL_10.pdf (Accessed
Oct, 2011).
- Psychotropic Expert Group. Therapeutic guidelines: psychotropic. Version 6. Melbourne: Therapeutic Guidelines Limited;
2008.
- Kessing V, Thomsen A, Mogensen U, Andersen P. Treatment with antipsychotics and the risk of diabetes in clinical
practice. Br J Psych 2010; 197:266–71.
- Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia:
a meta-analysis. Lancet 2009;373:31-41.
- National Institute for Clinical Excellence (NICE). Schizophrenia: core interventions in the treatment and management
of schizophrenia in adults in primary and secondary care (update). National Clinical Guideline Number 82. The British
Psychological Society & The Royal College of Psychiatrists, 2010. Available from: www.nice.org.uk (Accessed
Oct, 2011).
- Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for the Treatment of
Schizophrenia and Related Disorders. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines
for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry 2005;39(1-2):1-30.
- Kendall T. The rise and fall of the atypical antipsychotics. Br J Psych 2011;199:266-8.
- Novartis New Zealand Limited. Clozaril (clozapine). Medicine datasheet. 2011. Available from: www.medsafe.govt.nz (Accessed
Oct, 2011).
- Banarjee S. The use of antipsychotic medication for people with dementia: A time for action. An independent report
commissioned and funded by the Department of Health (England). October 2009. Available from: www.dh.gov.uk (Keyword
Bannerjee) (Accessed Oct, 2011).
- British National Formulary (BNF). BNF 62. London: BMJ Publishing Group and Royal Pharmaceutical Society of Great
Britain, 2011.
- Scottish Intercollegiate Guidelines Network (SIGN). Management of patients with dementia. A national clinical guideline.
SIGN, 2006. Available from: www.sign.ac.uk/pdf/sign86.pdf (Accessed
Oct, 2011).
- Monasterio E, McKean A. Off-label use of atypical antipsychotic medications in Canterbury, New Zealand. N Z Med J
2011;124(1336).
- Kmietowicz Z. Eli Lilly pays record $1.4bn for promoting off-label use of olanzapine. BMJ 2009;338:b217.
- Tanne J. Astra Zeneca pays $520m fine for off-label marketing. BMJ 2010;340:c2380.
- Wilkins C, Sweetsur P, Smart B, Griffiths R. Recent trends in illegal drug use in New Zealand, 2006-2010. Findings
from the 2006, 2007, 2008, 2009 and 2010 Illicit Drug Monitoring System (IDMS). Auckland: Social and Health Outcomes
Research and Evaluation (SHORE), School of Public Health, Massey University, 2011.
- Baldwin D, Anderson I, Nutt D, et al. Evidence based guidelines for the pharmacological treatment of anxiety disorders:
recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2005;19(6):567-96.
- National Prescribing Service Limited. Which treatment for what anxiety disorder? NPS News 2009;65. Available from: www.nps.org.au (Accessed
Oct, 2011).
- Gale C, Davidson O. Generalised anxiety disorder. BMJ 2007;334:579-81.
- Ravindran A, Al-Subaie A, Abraham G. Quetipaine: novel uses in the treatment of depressive and anxiety disorders.
Expert Opin Investig Drugs 2010;19(10):1187-204.
- Ruelaz Maher A, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications
for off-label uses in adults. JAMA 2011;306(12):1359-69.
- National Institute for Clinical Excellence (NICE). Guidance on the use of zaleplon, zolpidem and zopiclone for the
short-term management of insomnia. NICE, 2004. Available from: www.nice.org.uk (Accessed
Oct, 2011).
- Laine C, Goldmann D, Wilson JF. In the clinic: Insomnia. Ann Intern Med 2008; 148(1).
- Wine J, Sanda C, Caballero J. Effects of quetiapine on sleep in non-psychiatric and psychiatric conditions. Ann Pharmacother
2009;43(4):707-13.
- Monasterio E, Bhalla R, McKean A. Olanzapine-induced acute pancreatitis and new diabetes mellitus. 2011: In Print.
- Proietto J. Diabetes and Antipsychotic Drugs. Prescriber Update 2004;25(2):20-22.
- Sweetman SC. Martindale: The complete drug reference. 37th edition. Pharmaceutical Press, London, April 2011.
- Mylan New Zealand Limited. Olanzine & Olanzine-D. Medicine datasheet. 2011. Available from: www.medsafe.govt.nz (Accessed
Oct, 2011).