Key practice points:
- Patient characteristics, including co-morbidities, and the pharmacological properties of individual medicines should be considered before selecting a beta blocker for patients with heart failure, arrhythmias or other cardiovascular conditions. For example:
- Cardioselective beta blockers, e.g. bisoprolol, metoprolol, are less likely to cause bronchoconstriction than non-selective beta blockers, e.g. propranolol, and therefore may be preferred for patients with co-morbid respiratory disease. Cardioselective beta blockers are also less likely to cause cold extremities.
- Vasodilating beta blockers, e.g. carvedilol, are associated with fewer metabolic adverse effects and therefore may be preferred for patients with co-morbid type 2 diabetes (or those who are at increased risk). However, in practice, bisoprolol or metoprolol succinate is usually prescribed as these are cardioselective and generally dosed once daily, whereas carvedilol is dosed twice daily.
- Water-soluble beta blockers, e.g. atenolol, are less likely to cause central nervous system adverse effects (e.g. sleep difficulties) and therefore may be preferred for patients experiencing these effects with lipid-soluble beta blockers, e.g. metoprolol
- When initiating a beta blocker, slowly up-titrate the dose to minimise potential adverse effects. Beta blockers are generally well tolerated, but possible adverse effects depend on their properties (as detailed above) and include bradycardia, bronchospasm, hypotension, insulin resistance, fatigue, cold extremities and sexual dysfunction.
- Treatment with beta blockers is generally long-term, but withdrawal is sometimes appropriate. Beta blockers should be withdrawn slowly, ideally over several months, to prevent clinical deterioration and withdrawal symptoms, e.g. resting tachycardia.
- The optimal treatment duration post-myocardial infarction in patients with preserved ejection fraction and no regional wall motion abnormalities (RWMA) on echocardiogram remains uncertain. Evidence increasingly supports the withdrawal of beta blockers 12 months post-myocardial infarction in these patients who do not have other indications for treatment, e.g. heart failure, arrhythmias. Treatment beyond this period has not been associated with improved cardiovascular outcomes in this group.
This is a revision of a previously published article. What’s new for this update:
- A general article revision
- Metoprolol succinate use has decreased by approximately 15% since 2016, although it remains the most frequently prescribed beta blocker in New Zealand
- Beta blockers with intrinsic sympathomimetic activity (e.g. celiprolol and pindolol) are no longer available in New Zealand
- Section added on prescribing beta blockers to other patient groups, e.g. those with diabetes, those who are pregnant or breast feeding
- Revision of evidence on the treatment duration of beta blockers post-myocardial infarction
Beta blockers are one of the most frequently prescribed medicines in New Zealand and are indicated for a wide range of conditions. Cardiovascular indications such as heart failure and atrial fibrillation are the most common, but some beta blockers, e.g. propranolol, are also indicated for non-cardiovascular conditions, e.g. somatic symptoms of anxiety, migraine prophylaxis.1, 2
There is a disproportionate use of metoprolol succinate in New Zealand, a relatively cardioselective beta blocker, which is out of line with other similar countries such as Australia (see: “Metoprolol succinate remains the most frequently prescribed beta blocker in New Zealand”).3, 4 Rather than selecting the same beta blocker each time you prescribe, consider relevant patient characteristics, e.g. the presence of co-morbidities such as diabetes, the indications for use of each beta blocker and the pharmacological properties of each one when making your choice.
The pharmacology of beta blockers
Beta blockers are competitive antagonists of beta-adrenoceptors located throughout the autonomic nervous system, e.g. heart, bronchial and vascular smooth muscle, liver.5, 6 This prevents the actions of adrenaline and noradrenaline (i.e. the “fight or flight” response) on beta-adrenoceptors which results in a range of cardiovascular effects, including negative inotropy and chronotropy (i.e. decreased cardiac contractility and slowed heart rate), reduced cardiac output and decreased renin release.5, 7 Pharmacological differences, such as the type of beta-adrenoceptor selectivity, whether they are also selective for alpha-adrenoceptors and their lipid solubility, make each beta blocker, including their adverse effect profile, unique (Table 1).8, 9
Table 1. Properties of funded beta blockers currently available in New Zealand.1, 9, 10
| Beta blocker |
Beta-adrenoceptor selectivity |
Vasodilation |
Lipid solubility |
Excretion |
| Atenolol |
β1 selective |
No |
Low |
Renal |
| Bisoprolol |
β1 selective (strongly selective) |
No |
Yes |
Renal/hepatic |
| Carvedilol |
Non-selective (also alpha1-adrenoceptor selective) |
Yes |
Yes |
Hepatic |
| Labetalol |
Non-selective (also alpha1-adrenoceptor selective) |
Yes |
Yes |
Hepatic |
| Metoprolol |
β1 selective |
No |
Yes |
Hepatic |
| Nadolol |
Non-selective |
No |
Low |
Renal |
| Propranolol |
Non-selective |
No |
Yes |
Hepatic |
| Sotalol |
Non-selective |
No |
Low |
Renal |
Non-selective, cardioselective and vasodilating beta blockers
Beta blockers are largely classified according to their adrenoceptor binding affinities (Table 1), the degree of which varies between each medicine.10 There are three main types of beta-adrenoceptors:5, 6 ,10
- Beta1-adrenoceptors (75% of all beta-adrenoceptors), predominantly located in the heart
- Beta2-adrenoceptors, predominantly located in bronchial and vascular smooth muscle, but also uterine, liver and skeletal muscle
- Beta3-adrenoceptors, predominantly located in adipocytes (there is some evidence that beta3-adrenoceptors are also located in cardiomyocytes, however, their clinical relevance is uncertain).11 Research is ongoing into beta3-adrenoceptors as a target for the development of obesity medicines.12
Some beta blockers, e.g. carvedilol, also bind to alpha-adrenoceptors and prevent contraction of vascular smooth muscle.10
Non-selective beta blockers, e.g. propranolol, block beta1- and beta2-adrenoceptors equally.10, 13
Cardioselective beta blockers, e.g. bisoprolol, metoprolol, atenolol, have a greater affinity for beta1-adrenoceptors.5, 13 Bisoprolol is reported to be more cardioselective than metoprolol and atenolol.7, 11 There is, however, potential at higher doses for cardioselective beta blockers to also block beta2-adrenoceptors.9, 10
Vasodilating beta blockers, e.g. carvedilol, labetalol, reduce peripheral vascular resistance by binding to alpha-adrenoceptors causing vasodilation.9, 13 In addition to alpha1-blockade, carvedilol binds non-selectively to beta1- and beta2-adrenoceptors.9, 13
Some beta blockers, e.g. celiprolol and pindolol, have intrinsic sympathomimetic activity (ISA), which simultaneously block and stimulate beta-adrenoceptors causing less bradycardia and peripheral vasoconstriction.8 However, there are currently no beta blockers with ISA available in New Zealand.
Beta blockers can be water- or lipid-soluble
Water-soluble beta blockers, e.g. atenolol, nadolol, are excreted unchanged via the kidneys;9, 10 dose reductions may be necessary in patients with reduced renal function.1 Lipid-soluble beta blockers, e.g. carvedilol, metoprolol, propranolol, are metabolised in the liver and may be better tolerated by patients with reduced renal function.9, 10 Dose reductions may be necessary for patients with hepatic impairment who are taking lipid-soluble beta blockers, or consider switching to a water-soluble beta blocker; carvedilol should be avoided in patients with hepatic impairment.1 Bisoprolol is processed by both the liver and kidneys; dose reductions for patients with either renal or hepatic impairment are not usually required (unless in end-stage kidney or liver disease).1
Lipid-soluble beta blockers can penetrate through the blood brain barrier and cause more central nervous system-related adverse effects (e.g. sleep disturbances) than water-soluble beta blockers (see: “Water-soluble beta blockers less likely to cause central nervous system effects”).7, 13
Beta blockers can influence and interact with other medicines
All beta blockers can potentiate adverse effects such as bradycardia and hypotension when taken in combination with other medicines, e.g. diltiazem.1 Beta blockers that are metabolised by hepatic enzymes, e.g. metoprolol, may also interact with medicines that are metabolised via the same pathway.
The NZF interactions checker provides details on medicine interactions, including their clinical significance, available from: www.nzf.org.nz
Metoprolol succinate remains the most frequently prescribed beta blocker in New Zealand
Metoprolol succinate accounts for more than half of the beta blockers dispensed in New Zealand (Figure 1),4 and was the eighth most prescribed medicine overall in 2023.15 Although use remains high, it has reduced by approximately 15% since 2016.16 However, further efforts are needed to align prescribing with other similar countries such as Australia, where metoprolol succinate accounts for < 5% of beta blockers dispensed.3 Clinicians should individualise beta blocker prescribing as predominant use of one medicine leaves New Zealand vulnerable to supply issues.
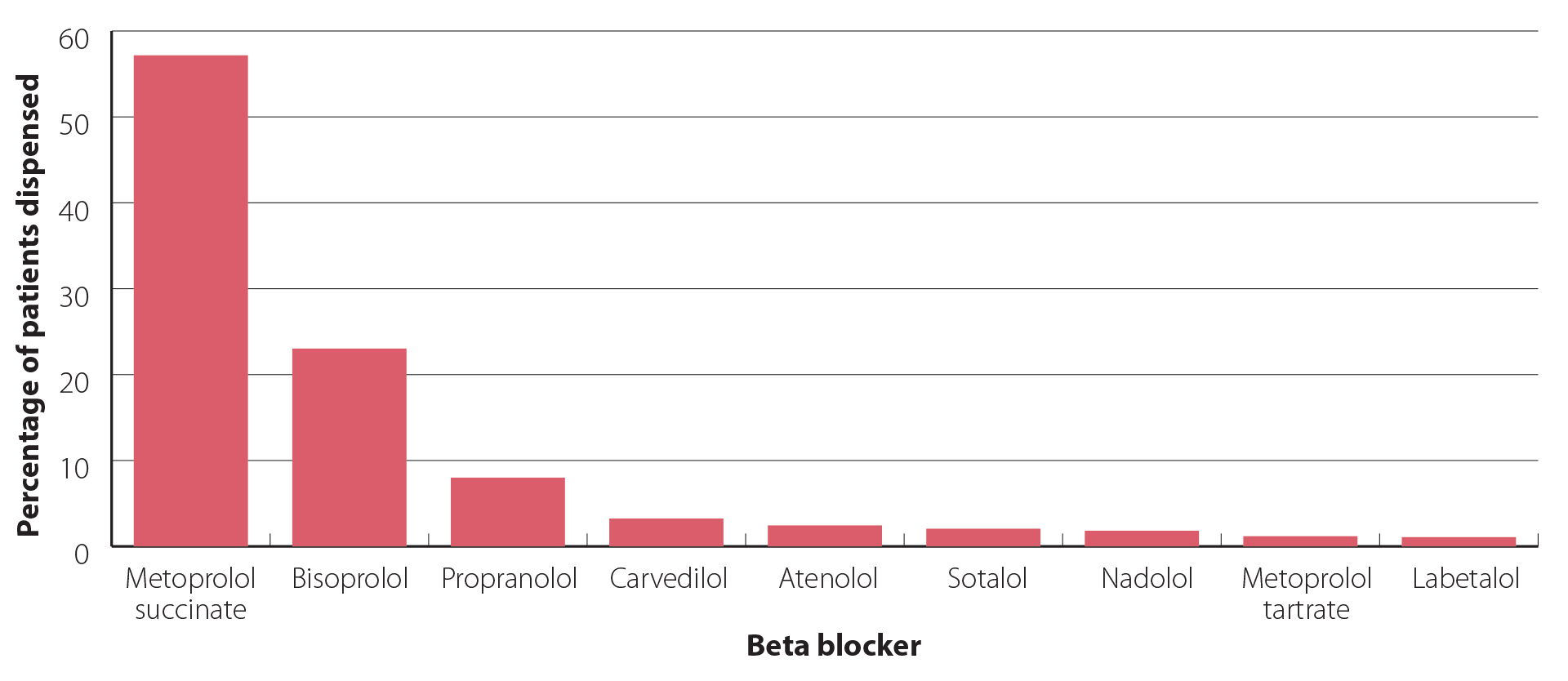
Figure 1. Percentage of patients dispensed each beta blocker in New Zealand in 2023.4
Why is metoprolol succinate prescribed so widely in New Zealand?
Metoprolol succinate is likely the beta blocker of choice among many prescribers in New Zealand because it has a wide range of cardiovascular indications, i.e. angina, arrhythmias, heart failure, myocardial infarction, hypertension, is relatively cardioselective and is usually dosed once daily (see: “The pharmacology of beta blockers”). However, there are other beta blockers that are also indicated for a wide range of cardiovascular conditions and are dosed once daily, e.g. bisoprolol, atenolol. Familiarity with a particular medicine and brand, in addition to historical funding and sole supply status (of the Betaloc brand) are also likely to have influenced prescribing habits.
 What is the difference between metoprolol succinate and metoprolol tartrate?
What is the difference between metoprolol succinate and metoprolol tartrate?
Metoprolol succinate and metoprolol tartrate are different salts of the same active ingredient.17 It is important not to confuse the two formulations of metoprolol when they are prescribed as they are not interchangeable; doses and indications differ.1 Metoprolol succinate is a modified-release formulation usually taken once daily, while most formulations of metoprolol tartrate are immediate-release taken at least twice or three times daily (there is one funded modified-release formulation available).1 Metoprolol tartrate immediate-release tablets can be crushed or formulated into a liquid for patients with swallowing difficulties.
Metoprolol tartrate is indicated for angina, arrhythmias, hypertension, hyperthyroidism (adjunct) and migraine prophylaxis. Metoprolol succinate has a wider range of indications: angina, adjunctive treatment in heart failure, arrhythmias, myocardial infarction, hypertension, hyperthyroidism and migraine prophylaxis.
The indications for beta blockers have shifted over the years. Originally, they were widely prescribed first line for hypertension and contraindicated for the treatment of heart failure. Now, beta blockers have a limited role in the management of hypertension and are routinely prescribed to patients with heart failure. The benefits of long-term beta blocker treatment post-myocardial infarction are also no longer as clear as they once were. Table 2 provides a summary of indications, recommendations and considerations for the use of beta blockers for cardiovascular conditions.
Table 2. Summary of indications, recommendations and considerations for the use of beta blockers for cardiovascular conditions in New Zealand.
| Indication |
Recommendation |
| Stable angina |
Beta blockers or calcium channel blockers are first-line medicines for the management of patients with stable angina.18 N.B. Calcium channel blockers should generally be avoided in patients with angina and HFrEF.19
All beta blockers are considered equally effective,19 although in practice, a cardioselective beta blocker (e.g. bisoprolol, metoprolol, atenolol) may be preferred as it is likely to provide the maximum effect with the minimum amount of adverse effects. |
| Arrhythmias |
A beta blocker is usually initiated for long-term rate control in patients with a cardiac arrythmia.20–23 A rate-limiting calcium channel blocker (diltiazem, verapamil) is also a suitable first-line option for rate control for patients with atrial fibrillation.20, 22, 23
Any beta blocker (apart from sotalol – see: “Arrhythmias: bisoprolol, metoprolol succinate or carvedilol preferred”) can be used for rate control but bisoprolol (unapproved indication), metoprolol succinate or carvedilol (unapproved indication) are usually preferred.14, 22, 24 In practice, bisoprolol is often trialled first as it is more cardioselective than metoprolol and may slow the heart rate slightly more than other beta blockers. |
| Heart failure |
International guidance is increasingly recommending that most patients with HFrEF should be established on the “four pillars of heart failure treatment” as early as possible.25, 26 This includes an ARNI, i.e. sacubitril + valsartan, beta blocker, MRA, e.g. spironolactone, eplerenone, and a SGLT-2 inhibitor, i.e. empagliflozin.25, 26 See: “Heart failure: prescribe bisoprolol, metoprolol succinate or carvedilol” for further information.
Bisoprolol, metoprolol succinate or carvedilol are the preferred beta blockers as they can reduce symptom severity, hospitalisation and mortality in people with HFrEF.7, 27 Patient-specific factors guide choice. In patients with heart failure that is associated with atrial fibrillation, there is evidence that carvedilol is superior to metoprolol succinate;40 however, in practice, bisoprolol or metoprolol succinate is usually prescribed to these patients as these are cardioselective and generally dosed once daily. Bisoprolol may also be the preferred choice for patients experiencing hypotension or dizziness with an ARNI and carvedilol.7 Bisoprolol may maintain pulmonary function and protect from myocardial injury to a greater extent than carvedilol.7 |
| Post-myocardial infarction |
A cardioselective beta blocker, e.g. bisoprolol, is usually prescribed to patients post-myocardial infarction, however, international guidelines do not recommend a specific beta blocker.11, 28
The optimal treatment duration of a beta blocker post-myocardial infarction in patients with preserved ejection fraction and no regional wall motion abnormalities (RWMA) on echocardiogram remains uncertain.29 Evidence increasingly supports the withdrawal of beta blockers one year post-myocardial infarction in these patients who do not have other indications for treatment, e.g. heart failure, arrhythmias, as treatment beyond this period has not been associated with improved cardiovascular outcomes.28, 30 A beta blocker is usually required indefinitely for patients with reduced left ventricular ejection fraction or evidence of myocardial damage as demonstrated by regional wall motion abnormalities on echocardiogram.28 |
| Hypertension |
Beta blockers are no longer first line for the management of hypertension unless there is a specific clinical reason for their use, e.g. co-morbid atrial fibrillation or heart failure, female of reproductive age.31
All beta blockers are considered equally effective for hypertension.7, 13, 32 In practice, a cardioselective or vasodilating beta blocker is usually prescribed. |
| Co-morbidities and considerations |
| Renal impairment |
Consider dose adjustment for water-soluble beta blockers or a lipid-soluble beta blocker may be better tolerated.1 Dose adjustments of bisoprolol are not usually required in renal impairment (unless impairment is severe).1 |
| Hepatic impairment |
Consider dose adjustment for lipid-soluble beta blockers (e.g. metoprolol, propranolol)1 or switch to a water-soluble beta blocker. Carvedilol should be avoided.1 Dose adjustments of bisoprolol are not usually required in hepatic impairment (unless impairment is severe).1 |
| Asthma |
Beta blockers are generally avoided in patients with asthma, however, if a beta blocker is required, prescribe a cardioselective beta blocker6, 14 |
| COPD |
Cardioselective beta blockers are recommended31, 33 |
| Diabetes |
Evidence suggests that carvedilol may be the preferred beta blocker for patients with cardiovascular disease and diabetes (or who are at increased risk) as it does not adversely affect glycaemic control and may improve insulin sensitivity.2, 14 However, in practice, bisoprolol or metoprolol succinate is usually prescribed to these patients as these medicines are cardioselective and generally dosed once daily, whereas carvedilol is dosed twice daily and if there are issues with adherence, this would reduce the benefit. |
| Pregnancy |
Labetalol is usually first-line if a beta blocker is indicated during pregnancy as it is generally associated with the lowest risk of adverse neonatal effects.34, 35 Metoprolol or bisoprolol may be a suitable alternative if required.34 |
| Breast feeding |
Carvedilol, labetalol, metoprolol and propranolol are present in breast milk in low quantities but this is not expected to affect infants.1 Atenolol, sotalol and nadolol should generally be avoided while breast feeding (particularly for newborns and pre-term infants or if taking high doses) as they are excreted in breast milk in higher quantities.1 |
| Other considerations |
Cardioselective beta blockers (e.g. bisoprolol, metoprolol, atenolol) are less likely to cause cold extremities36
Water-soluble beta blockers (e.g. atenolol) are less likely to cause central nervous system effects such as sleep disturbances7, 13
Bisoprolol is less likely to cause sexual dysfunction compared to other beta blockers7, 14, 36 |
N.B. When “metoprolol” is specified, either the succinate or tartrate salt can be prescribed. In practice, metoprolol succinate is often preferred as it is usually dosed once daily (see: “What is the difference between metoprolol succinate and metoprolol tartrate”).
HFrEF = heart failure with reduced ejection fraction, ARNI = angiotensin-receptor neprilysin inhibitor, MRA = mineralocorticoid receptor antagonist, SGLT-2 inhibitor = sodium-glucose co-transporter 2 inhibitor, ACE inhibitor = angiotensin-converting enzyme inhibitor, ARB = angiotensin-II receptor blocker
Stable angina: preference and co-morbidities determine treatment choice
Beta blockers or calcium channel blockers are first-line medicines for the management of patients with stable angina.18 There is no evidence that beta blockers are superior to calcium channel blockers or that one beta blocker is superior to another.19 Choice of a beta blocker or calcium channel blocker is largely determined by the presence of co-morbidities (e.g. calcium channel blockers should generally be avoided in patients with HFrEF), contraindications, the patient’s preference for dose frequency and their likely adherence to treatment.18, 19 In practice, a cardioselective beta blocker is likely to provide the maximum effect with the minimum amount of adverse effects.
For further information on the management of stable angina, see: bpac.org.nz/BPJ/2011/october/angina.aspx (N.B. This article is currently undergoing revision and a new version will be published in 2024.)
Arrhythmias: bisoprolol, metoprolol succinate or carvedilol preferred
A beta blocker is usually initiated for long-term rate control in patients with a cardiac arrhythmia, including atrial fibrillation and ventricular tachycardia.20–23 A non-dihydropyridine calcium channel blocker, e.g. diltiazem, verapamil, is also a suitable first-line option for rate control in patients with atrial fibrillation in the absence of HFrEF.20, 22, 23
Any beta blocker (apart from sotalol – see below) may be prescribed to a patient for rate control, but bisoprolol (unapproved indication), metoprolol succinate and carvedilol (unapproved indication) are usually preferred.14, 22 In practice, bisoprolol is often trialled first as it is more cardioselective than metoprolol and may slow the heart rate slightly more than other beta blockers.
Sotalol causes beta blockade at low doses, e.g. 40 mg, twice daily, however, this dose is often insufficient for rate control in patients with atrial fibrillation. At higher doses, i.e. ≥ 80 mg, twice daily, sotalol has Class III antiarrhythmic effects and is used for rhythm control in this setting.21, 37 However, use has declined since the SWORD (Survival With ORal D-sotalol) study in the 1990s was terminated after sotalol was associated with a higher rate of sudden death when administered to patients with reduced left ventricular ejection fraction after myocardial infarction.21 In practice, sotalol is used infrequently for rhythm control as it can prolong the QT interval (torsadogenic), is renally excreted which is undesirable in an elderly population, and other rhythm control strategies are considered to be more effective.37 Alternative medicines for rhythm control include flecainide and amiodarone.1
For further information on the management of atrial fibrillation in primary care, see: bpac.org.nz/2024/af.aspx
Heart failure: prescribe bisoprolol, metoprolol succinate or carvedilol
International guidance is increasingly recommending that most patients with heart failure with reduced ejection fraction (HFrEF) should be established on the “four pillars of heart failure treatment” as early as possible.25, 26 This includes an angiotensin-receptor neprilysin inhibitor (ARNI, i.e. sacubitril + valsartan), beta blocker, mineralocorticoid receptor antagonist (MRA, e.g. spironolactone, eplerenone) and a sodium-glucose co-transporter 2 inhibitor (SGLT-2, i.e. empagliflozin).25, 26 The practical capacity to achieve this combination of medicines will depend on patient co-morbidities and funding restrictions, but should be encouraged where possible to decrease mortality and hospitalisation,38 and potentially support cardiac structure recovery.
In a primary care setting, patients with HFrEF are usually initiated on a beta blocker and an angiotensin-converting enzyme (ACE) inhibitor or angiotensin-II receptor blocker (ARB); a loop diuretic is used initially to reduce fluid overload.39 If the patient remains symptomatic despite maximum tolerated doses, treatment is escalated by adding either spironolactone (or this may be added initially for patients with severe symptoms) or switching the ACE inhibitor/ARB to an ARNI (if the patient meets Special Authority criteria for funded treatment or chooses to self-fund). Patients with HFrEF and co-morbid type 2 diabetes will also be eligible for funded empagliflozin treatment if they meet Special Authority criteria (others may choose to self-fund).
In terms of beta blocker, bisoprolol, metoprolol succinate and carvedilol have been shown to reduce symptom severity, hospitalisation and mortality in people with HFrEF;7, 27 the choice of which is guided by patient-specific factors. In patients with heart failure that is associated with atrial fibrillation, there is evidence that carvedilol is superior to metoprolol succinate;40 however, in practice, bisoprolol or metoprolol succinate is often prescribed to these patients as these medicines are cardioselective and generally dosed once daily. Bisoprolol may also be the preferred choice for patients experiencing hypotension or dizziness with combination treatment of an ARNI and carvedilol.7 Evidence from a small study suggests that in patients with HFrEF, bisoprolol may maintain pulmonary function and protect from myocardial injury to a greater extent than carvedilol.7 The dose should be slowly up-titrated to the target or maximum tolerated dose.7
Some patients with heart failure with preserved ejection fraction (HFpEF) may be prescribed a beta blocker if they have other cardiovascular co-morbidities, e.g. atrial fibrillation.41
For further information on the management of heart failure, see: bpac.org.nz/2022/heart-failure.aspx
Post-myocardial infarction: a beta blocker will usually be initiated in secondary care
A beta blocker is usually given acutely in secondary care as first-line treatment post-myocardial infarction, alongside an ACE inhibitor, antiplatelets and a statin (unless they have a completely normal echocardiogram), to decrease infarct size, reduce the risk of ventricular arrhythmias, and in the long-term, to prevent dysfunctional ventricular remodelling and heart failure.11, 28 In practice, a cardioselective beta blocker is usually prescribed, however, international guidelines do not specify which beta blocker is preferred.11, 28
Beta blocker withdrawal may be appropriate 12 months post-myocardial infarction
Patients are generally reviewed with an echocardiogram three months post-myocardial infarction. In those with preserved ejection fraction and no negative cardiac remodelling, some cardiology centres may discontinue the beta blocker. Others may recommend that patients continue beta blocker treatment for at least 12 months post-myocardial infarction before reviewing again.28, 29 The optimal treatment duration with a beta blocker post-myocardial infarction in patients with preserved ejection fraction and no regional wall motion abnormalities (RWMA) on echocardiogram, however, remains uncertain and recommendations differ between cardiologists and cardiology centres.29
The two main reasons why the optimal treatment duration with a beta blocker post-myocardial infarction is uncertain are:29, 42
- Frequent, more advanced and quicker access to reperfusion techniques and the routine use of statins and anti-platelet medicines post-myocardial infarction mean that patients now gain less benefit from the use of beta blockers than they did before these treatments were commonplace. The modest benefits of beta blockers post-myocardial infarction were only apparent in the pre-revascularisation era where patients had larger myocardial infarctions and reduced ejection fractions.
- There are limited recent prospective randomised controlled trials assessing the long-term benefits of beta blockers in patients with uncomplicated myocardial infarction
Evidence increasingly supports the withdrawal of beta blockers one year post-myocardial infarction in patients with preserved ejection fraction (≥ 50%), no regional wall motion abnormalities on echocardiogram and no other indications for treatment, e.g. heart failure, arrhythmias, as treatment beyond this period has not been associated with improved cardiovascular outcomes.28, 30 A study published in 2024 (including patients from New Zealand) found that in patients post-myocardial infarction with preserved ejection fraction, long-term beta blocker treatment (median follow-up 3.5 years) did not reduce mortality from any cause or new myocardial infarction.43
A beta blocker is usually required indefinitely for patients with reduced left ventricular ejection fraction or evidence of myocardial damage as demonstrated by regional wall motion abnormalities on echocardiogram.28
For information on the management of acute coronary syndromes, see: bpac.org.nz/BPJ/2015/April/coronary.aspx
Hypertension: beta blockers no longer first line
Beta blockers are usually only prescribed to patients with hypertension when there is a specific clinical reason for their use, as other antihypertensive medicines (i.e. ACE inhibitors, ARBs, thiazide/thiazide-like diuretics and calcium channel blockers) are generally associated with better outcomes, e.g. a greater reduction in stroke risk.31, 44 Clinical scenarios where a beta blocker may be indicated for a patient with hypertension, either first line as monotherapy or at any stage in combination treatment, include patients with co-morbid atrial fibrillation, coronary artery disease, heart failure or in females of reproductive age (renin-angiotensin system blockers and diuretics should be avoided in patients with hypertension who are planning pregnancy or who are pregnant).31, 44
The choice of beta blocker should be based on the presence of co-morbidities and the patient’s preference for frequency of dosing and their likely adherence to treatment. In practice, a cardioselective or vasodilating beta blocker is generally selected, however, there is no evidence that one beta blocker is superior to another.7, 13, 32 Carvedilol may be the preferred beta blocker for patients with hypertension and diabetes, as there is evidence that it improves insulin sensitivity and glycaemic control, but adherence to twice daily dosing could be an issue (see: “Prescribing beta blockers to other patient groups”).13 Labetalol is usually prescribed if a beta blocker is indicated for patients with hypertension who are pregnant,31, 35 as it generally is associated with the lowest risk of adverse neonatal effects compared to other beta blockers.34
For further information on the management of hypertension, see: bpac.org.nz/2023/hypertension.aspx
For information on the management of hypertension in pregnancy, see: www.tewhatuora.govt.nz/publications/diagnosis-and-treatment-of-hypertension-and-pre-eclampsia-in-pregnancy-in-aotearoa-new-zealand/
Beta blockers are generally initiated at a low dose and up-titrated to the target or maximum tolerated dose, but this can sometimes depend on the cardiovascular indication and patient-related factors.1 For example, in atrial fibrillation the starting dose depends on the patient’s heart rate and co-morbidities, e.g. 23.75 mg metoprolol succinate, once daily, may be an appropriate starting dose for a patient with a moderately elevated heart rate, but a higher initial dose, e.g. 47.5 mg, may be required for those with a significantly elevated heart rate.*
*Recommended dose differs from the New Zealand Formulary for “arrhythmias”, which lists the lower threshold as 95 mg, once daily.1 Lower doses, e.g. 23.75 mg, can be a suitable starting point for select patients with atrial fibrillation as they are still often effective and reduce the risk of adverse effects.
Refer to the NZF for individual beta blocker dosing regimens: nzf.org.nz/nzf_1108
Beta blockers are not usually recommended in patients with asthma
Beta blockers are generally avoided in people with asthma, as they can cause bronchoconstriction and be associated with airway hyper-responsiveness, trigger exacerbations and reduce the effectiveness of bronchodilators.6 If a beta blocker is needed for a patient with asthma, prescribe a cardioselective beta blocker, e.g. bisoprolol, metoprolol, at a low dose, as these are better tolerated than non-selective beta blockers and less likely to cause harm.6, 14 There is some evidence that cardioselective beta blockers in people with a cardiovascular condition and co-morbid asthma do not increase exacerbations, reduce airway function or worsen quality of life.6 At high doses, however, they can still block beta2-adrenoceptors and cause adverse respiratory effects; use with caution if required.6
Prescribe cardioselective beta blockers to patients with COPD
Clinicians may be reluctant to prescribe beta blockers to patients with co-morbid COPD due to concerns of adverse pulmonary effects, however, studies have demonstrated that beta blockers are safe when used in this context.2, 31 The risk/benefit assessment for a person with cardiovascular disease and COPD differs to a person with cardiovascular disease and asthma. People with COPD are at greater risk of ischaemic heart disease and other cardiovascular diseases (e.g. heart failure) than people with asthma, and would benefit more from the use of beta blockers.7 The safety margin of beta blockers is understood to be wider in people with COPD than asthma.31
If a beta blocker is indicated for a patient with COPD, a cardioselective beta blocker, e.g. bisoprolol, metoprolol, at a low dose is usually most appropriate.7, 31, 33 A systematic review and meta-analysis of 49 studies, including more than 670,000 people with cardiovascular disease and COPD found evidence that beta blockers decrease mortality (both non-selective and cardioselective) and reduce COPD exacerbations (only cardioselective).31
Prescribing beta blockers to other patient groups
 Diabetes or at increased risk of diabetes. Diabetes is a common co-morbidity associated with cardiovascular disease, e.g. ischaemic heart disease, heart failure.2, 14 Beta blockers are generally safe to use in patients with type 2 diabetes (or who are at increased risk); vasodilating beta blockers, i.e. carvedilol, are associated with fewer adverse metabolic effects.2, 14 There is some evidence that carvedilol improves insulin sensitivity and does not adversely affect glycaemic control.2, 14 There is also minimal effect on lipid metabolism and weight gain.9 However, in practice, bisoprolol or metoprolol succinate is usually prescribed to a patient with cardiovascular disease and type 2 diabetes (or who is at increased risk) as these medicines are cardioselective and generally dosed once daily, whereas carvedilol is dosed twice daily and if there are issues with adherence, this would reduce the benefit. Non-selective beta blockers (e.g. propranolol) should generally be avoided in patients at risk of diabetes at they may contribute to the development of diabetes, for example by increasing lipid levels.2, 44 Prescribe beta blockers with caution to patients with type 1 diabetes or who are taking insulin, as beta blockers can mask symptoms or signs of hypoglycaemia.9, 44
Diabetes or at increased risk of diabetes. Diabetes is a common co-morbidity associated with cardiovascular disease, e.g. ischaemic heart disease, heart failure.2, 14 Beta blockers are generally safe to use in patients with type 2 diabetes (or who are at increased risk); vasodilating beta blockers, i.e. carvedilol, are associated with fewer adverse metabolic effects.2, 14 There is some evidence that carvedilol improves insulin sensitivity and does not adversely affect glycaemic control.2, 14 There is also minimal effect on lipid metabolism and weight gain.9 However, in practice, bisoprolol or metoprolol succinate is usually prescribed to a patient with cardiovascular disease and type 2 diabetes (or who is at increased risk) as these medicines are cardioselective and generally dosed once daily, whereas carvedilol is dosed twice daily and if there are issues with adherence, this would reduce the benefit. Non-selective beta blockers (e.g. propranolol) should generally be avoided in patients at risk of diabetes at they may contribute to the development of diabetes, for example by increasing lipid levels.2, 44 Prescribe beta blockers with caution to patients with type 1 diabetes or who are taking insulin, as beta blockers can mask symptoms or signs of hypoglycaemia.9, 44
 Pregnancy and breast feeding. Labetalol is the first-line beta blocker when indicated for a patient with cardiovascular disease (e.g. hypertension) who is pregnant as it is generally associated with the lowest risk of adverse neonatal effects.34, 35 Metoprolol or bisoprolol may be considered as a second-line option.34 Other beta blockers, e.g. atenolol, are usually avoided during pregnancy as these have been associated with fetal complications, e.g. pre-term birth, growth restriction, structural abnormalities.1, 34 If a beta blocker is taken close to delivery, infants will be monitored for symptoms and signs of beta blockade, e.g. bradycardia, hypotension (and alpha blockade if labetalol or carvedilol is taken).
Pregnancy and breast feeding. Labetalol is the first-line beta blocker when indicated for a patient with cardiovascular disease (e.g. hypertension) who is pregnant as it is generally associated with the lowest risk of adverse neonatal effects.34, 35 Metoprolol or bisoprolol may be considered as a second-line option.34 Other beta blockers, e.g. atenolol, are usually avoided during pregnancy as these have been associated with fetal complications, e.g. pre-term birth, growth restriction, structural abnormalities.1, 34 If a beta blocker is taken close to delivery, infants will be monitored for symptoms and signs of beta blockade, e.g. bradycardia, hypotension (and alpha blockade if labetalol or carvedilol is taken).
Carvedilol, labetalol, metoprolol and propranolol can be present in breast milk in low quantities but this is not expected to affect infants.1 Atenolol, sotalol and nadolol should generally be avoided while breast feeding (particularly for newborns and pre-term infants or if taking high doses) as these can be present in breast milk in higher quantities.1 Infants ingesting breast milk from a mother taking a beta blocker should be monitored for symptoms and signs of potential toxicity, e.g. bradycardia, hypoglycaemia, hypotension, poor suckling (and alpha blockade if labetalol or carvedilol is taken).1
Adverse effects of beta blockers vary depending on their properties, e.g. beta-adrenoceptor selectivity, although they are generally well tolerated.8, 19 Possible adverse effects include bradycardia, bronchospasm, hypotension, insulin resistance, fatigue, cold extremities, depression and sexual dysfunction.13, 19 Gradual up-titration to the maintenance dose may improve patient tolerance and adherence to treatment.
Cardioselective beta blockers less likely to cause peripheral vasoconstriction
Cardioselective beta blockers, e.g. bisoprolol, metoprolol, are less likely to cause cold extremities than non-selective beta blockers.36 If cold extremities are a concern for a patient while taking a non-selective beta blocker, consider whether it is appropriate to either lower the dose, switch to a cardioselective beta blocker or add a dihydropyridine calcium channel blocker (e.g. amlodipine) to their treatment regimen.
Water-soluble beta blockers less likely to cause central nervous system effects
Vivid dreams, nightmares and in rare cases, hallucinations, can be caused by lipid-soluble beta blockers crossing the blood brain barrier.7, 13 In contrast, central nervous system effects are less likely to occur with water-soluble beta blockers.13 If a patient experiences intolerable central nervous system effects that cannot be managed with a dose reduction, consider whether switching to a water-soluble beta blocker, e.g. atenolol, would be appropriate.
Bisoprolol less likely to cause sexual dysfunction
The management of erectile dysfunction in patients taking beta blockers is complicated by the possibility of overlapping causes.7, 14 Beta blocker-associated erectile dysfunction appears to occur in a dose-dependent manner and is reportedly more frequent with non-selective beta blockers.14 Bisoprolol (and nebivolol - not available in New Zealand) is less likely to cause erectile dysfunction than other beta blockers, therefore, is preferred for patients experiencing erectile dysfunction.7, 14, 36
Phosphodiesterase type-5 inhibitors, e.g. sildenafil, can be prescribed to some patients with cardiovascular disease, e.g. heart failure, who experience erectile dysfunction with beta blocker use.14 Sildenafil should be avoided in patients with a recent history of stroke, unstable angina or myocardial infarction and in patients taking nitrates (use with caution in patients taking alpha blockers).1, 39
Treatment with beta blockers is generally long-term, but it should not be regarded as indefinite in all patients. In some clinical situations it may be necessary to withdraw treatment (either temporarily or completely) or reduce the dose, e.g. if adverse effects are intolerable or result in adverse sequalae such as leg ulcers from vasoconstriction. In the long-term, the emergence of co-morbidities as people age may make management more complex, and it is appropriate to periodically review and discuss the benefits and risks of treatment with each patient who is taking a beta blocker.
Stopping treatment: go slow to get low
Beta blockers should be withdrawn slowly to prevent clinical deterioration and withdrawal symptoms, which in some cases can be severe, e.g. ischaemic cardiac symptoms even in those with no history of coronary heart disease.19, 45
There are no specific guidelines for withdrawing beta blockers. A pragmatic approach is to reduce the dose over several months, e.g. taper a twice daily dose to once daily for one month, and then further reduce the dose to every second day for another month, before stopping treatment completely. The dose can be halved each week for patients who need a more rapid withdrawal.45 Monitor the patient’s heart rate and blood pressure during the dose tapering process.





