Published: 27 January 2023 | Updated: 5 June 2025
What's changed?
5 June 2025
Keypoints from ESC European Society of Cardiology (ESC) 2024 guidelines added
23 May 2024
Table 1 revised and updated
29 April 2024
new section added on cardiovascular-kidney-metabolic risk
10 August 2023
update box added regarding the European Society of Hypertension (ESH) 2023 guidelines
Update:
European Society of Cardiology (ESC) 2024 guidelines for the management of elevated blood pressure and hypertension
New guidelines from ESC may have implications for the management of people with hypertension in New Zealand.
Key points from ESC 2024 include:
- Hypertension continues to be defined as blood pressure (BP) ≥140/90 mmHg. However, a new category of “elevated BP” is introduced, defined by BP 120 – 139/70 – 89 mmHg:
- These patients are also considered to have an increased risk of cardiovascular events
- Patients who also have moderate or severe chronic kidney disease, established cardiovascular disease, diabetes or familial hypercholesterolemia, should be advised to undertake three months of lifestyle interventions (see below). If BP remains elevated, antihypertensive treatment is recommended.
- There is a stronger emphasis on use of out-of-office BP measurement than in previous guidelines (to exclude white-coat and masked hypertension). However, it is acknowledged that this approach is not always feasible. BP should be measured using a validated and calibrated device, and a consistent approach applied to measurements for each patient.
- Dual antihypertensive treatment continues to be recommended as the initial management strategy for most patients with hypertension
- NEW: A systolic BP target range of 120 – 129 mmHg is recommended for most patients receiving antihypertensive treatment. A more lenient target should only be considered in select circumstances (e.g. pre-existing symptomatic orthostatic hypotension, aged ≥ 85 years, moderate-to-severe frailty, limited life expectancy) or if treatment is not tolerated.
- This is described as a “paradigm shift from prior European guidelines” (including the 2018 ESC/ESH Hypertension Guidelines, the 2021 ESC Prevention Guidelines, and the 2023 ESH Hypertension Guidelines) which advocated for a two-step approach, i.e. first aiming for < 140/90 mmHg, then if tolerated aim for < 130/80 mmHg.
- In general, aim for a diastolic BP target of 70 – 79 mmHg. If the patient has a systolic BP at or below target (120 – 129 mmHg) but their diastolic BP is not at target (i.e. it is ≥ 80 mmHg), consider intensifying antihypertensive treatment to achieve a diastolic BP of 70 – 79 mmHg to reduce CVD risk
- The guidelines expand on advice for lifestyle options to help lower BP and CBD risk, including:
- Moderate intensity aerobic exercise ≥ 150 min/week (≥ 30 min, 5 – 7 days/week) or 75 minutes of vigorous intensity aerobic exercise per week over three days, ideally complemented with low- or moderate-intensity dynamic or isometric resistance training (2 – 3 times/week).
- Mediterranean or DASH diet. Restrict free sugar consumption, particularly sugar-sweetened beverages, to a maximum of 10% of energy intake.
- Device-based therapies (catheter-based renal denervation) may be considered in some patients with treatment resistant hypertension
McEvoy JW, McCarthy CP, Bruno RM, et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. European Heart Journal 2024;45:3912–4018. doi:10.1093/eurheartj/ehae178.
This article will be revised in time to reflect these changes. For now, refer to the Hypertension management B-QuiCK for up to date blood pressure treatment targets for adults.
Key practice points:
- The line separating blood pressure measurements from being “normotensive” and “hypertensive” is not clear-cut. Consider any elevated readings in the context of a patient’s overall cardiovascular disease (CVD) risk. The balance between these two factors will influence subsequent management decisions.
- Consider using 24-hour ambulatory or at-home monitoring to confirm persistently elevated clinic blood pressure readings, if resources are available
- Comprehensive clinical assessment in patients with newly identified hypertension should also include evaluation of urine albumin:creatinine ratio (ACR) and eGFR as impaired kidney function is strongly related to increased CVD risk
- Any patient with persistently elevated blood pressure readings should be encouraged to make lifestyle changes, e.g. weight loss, increased exercise, dietary changes including reducing sodium intake, limiting alcohol consumption, smoking cessation. Early adoption of meaningful changes could delay or prevent the need for antihypertensive medicines later in life. However, this may not be achievable for all patients.
- For patients with severe hypertension (e.g. ≥ 160/100 mmHg), antihypertensive treatment should be initiated immediately, in addition to lifestyle changes, regardless of the patient’s CVD risk (although CVD risk should still be calculated)
- For all other patients with a blood pressure persistently ≥ 130/80 mmHg, treatment decisions should be based on their five-year CVD risk calculated using New Zealand primary prevention equations:
- CVD < 5%: antihypertensive treatment is not recommended; proceed with lifestyle changes
- CVD 5 – 15%: consider antihypertensive treatment if blood pressure measurements are ≥ 140/90 mmHg, in addition to lifestyle changes
- CVD ≥ 15%: antihypertensive treatment is recommended, in addition to lifestyle changes
- Angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), calcium channel blockers and thiazide/thiazide-like diuretics are all first-line antihypertensives:
- The choice of antihypertensive and combination of treatment depends on patient co-morbidities (e.g. diabetes, renal impairment) and other characteristics (e.g. pregnancy)
- Beta-blockers are no longer first-line unless specifically indicated for a co-morbidity
- For most patients, initial treatment with two low-dose antihypertensives together (i.e. dual antihypertensive treatment) is a reasonable approach as this provides a more significant blood pressure-lowering effect compared with high dose monotherapy, while also reducing the risk of adverse effects
- Initial monotherapy remains appropriate for certain patient groups, e.g. those within 20/10 mmHg of their blood pressure target, committing to major lifestyle changes, elderly (e.g. aged ≥ 80 years) or frail
- Initial treatment in patients with hypertension and significant proteinuria or chronic kidney disease (CKD) should ideally include either an ACE inhibitor or ARB at the maximum tolerated dose as this optimises their antiproteinuric effect and delivers the most significant reduction in CVD/CKD risk (multiple antihypertensives are still often ultimately required to achieve blood pressure targets)
- Blood pressure targets should be individualised according to the patient’s CVD risk, co-morbidities and treatment objectives
- For patients not achieving blood pressure targets despite the use of two antihypertensives, the doses of existing medicines can be increased if they are close to their goal, or a third antihypertensive can be added if not
- If targets are still not being achieved despite use of three antihypertensives, reassess adherence to medicine and lifestyle changes as well as possible secondary causes, prior to considering the addition of another medicine, e.g. spironolactone or a beta-blocker. N.B. 24-hour ambulatory or at-home monitoring should also be strongly considered at this stage to confirm the patient’s true blood pressure, if not already completed.
This is a revision of a previously published article. What’s new for this update:
- A general article revision
- Information added on the relationship between hypertension and chronic kidney disease
- Changes to the recommendations for initiating antihypertensives based on a patient’s five-year CVD risk rather than blood pressure measurements in isolation
- Updated information on funded and available antihypertensive options
- Changes to the approach for initiating treatment, i.e. many people should be initiated on two low-dose antihypertensives rather than starting with a single antihypertensive and increasing the dose
- Discussion on individualising blood pressure targets
- Review of the conflicting evidence for night-time versus morning antihypertensive dosing
Hypertension is a common incidental clinical finding in primary care.1 While transient increases in blood pressure are normal and can occur for a variety of reasons, persistent elevation represents an important modifiable risk factor for many conditions including stroke, myocardial infarction, heart failure, atrial fibrillation, kidney disease and cognitive decline.1, 2 However, most people with hypertension are asymptomatic, giving rise to the moniker “silent killer” due to its insidious, chronic and progressive nature.3
International guidelines vary in the thresholds used to define hypertension.1, 4 ,5 While it has previously been common practice in New Zealand to consider any clinic blood pressure ≥ 140/90 mmHg as being “hypertensive”, blood pressure measurements alone are now considered insufficient to define and guide the management of hypertension in primary care.6 Blood pressure has a normal distribution across the general population and the cardiovascular disease (CVD) risk associated with increasing measurements is continuous.4 If additional factors are present that elevate cardiovascular risk further, a patient is more likely to experience a cardiovascular event, even if their blood pressure is within 130 – 139/85 – 89 mmHg:4, 5 the line between normotension and hypertension is therefore not clear-cut.
 Risk factors for hypertension include:5 age ≥ 65 years, male sex, excess body weight, sedentary lifestyle, kidney dysfunction, diabetes, high LDL-C/triglycerides, sustained increased resting heart rate (> 80 beats/min), a personal or family history of cardiovascular disease or hypertension (genetics), early-onset menopause, smoking (current or past), and psychosocial or socioeconomic factors.
Risk factors for hypertension include:5 age ≥ 65 years, male sex, excess body weight, sedentary lifestyle, kidney dysfunction, diabetes, high LDL-C/triglycerides, sustained increased resting heart rate (> 80 beats/min), a personal or family history of cardiovascular disease or hypertension (genetics), early-onset menopause, smoking (current or past), and psychosocial or socioeconomic factors.
Hypertension is prevalent yet undertreated in New Zealand
The age-standardised mean systolic blood pressure in people in New Zealand is increasing due to a range of factors, including higher rates of obesity and sedentary lifestyles, as well as the increased consumption of foods containing a high content of fat, sugar and salt.7 Hypertension is often under-treated; Māori, Pacific and Asian peoples with hypertension have significantly lower rates of antihypertensive use compared with European/Other peoples (Figure 1), a trend which has remained consistent over time.8 While not all patients with hypertension require pharmacological treatment (see: “When to initiate antihypertensive medicines”), these disparities need to be recognised and addressed across primary care to achieve more equitable health outcomes.
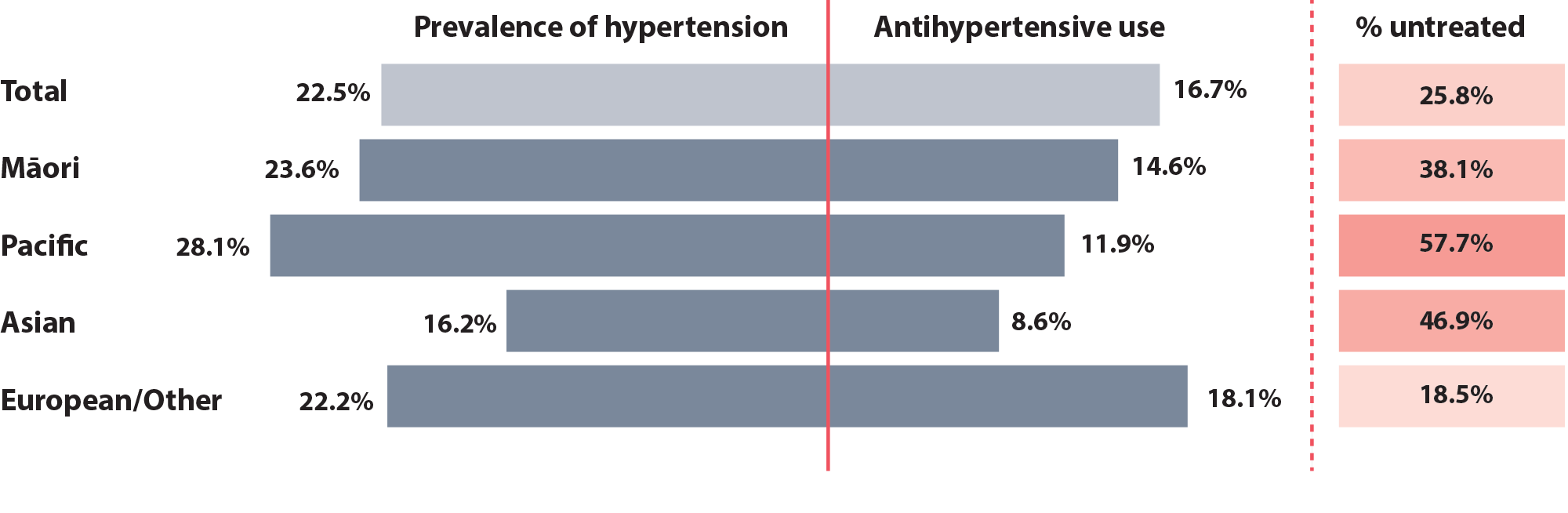
Figure 1. Prevalence of hypertension versus rates of antihypertensive use in New Zealand by ethnicity.8 Data obtained from the 2020/21 Ministry of Health New Zealand Health Survey. The percentage of patients not taking an antihypertensive was calculated using the datasets for “Raised blood pressure (measured)” and “High blood pressure (medicated)”.
Treatment for hypertension often involves lifelong exposure to multiple medicines and their potential adverse effects.5 Therefore, it is essential that hypertension is accurately diagnosed in primary care, but this can be challenging as most patients with hypertension are asymptomatic.
If a patient has a clinic blood pressure measurement ≥ 130/80 mmHg a clinical evaluation should be conducted to:
- Confirm the elevated blood pressure
- Assess CVD risk (including evaluation of kidney function)
- Determine if any end organ damage has occurred (including CKD)
- Identify any causes of secondary hypertension
1. Confirming elevated blood pressure
To achieve a more accurate assessment, it is recommended that two or more blood pressure measurements are taken, at least two minutes apart. Ideally, an additional measurement should also be taken in the patient’s other arm in case there is a significant difference.5, 9 Consistent systolic blood pressure differences ≥ 10 mmHg between arms are associated with an increased risk of cardiovascular disease.5
If blood pressure measurements are elevated at a single appointment, another reading should ideally be taken at a separate appointment on a different day to confirm a diagnosis of hypertension.9 N.B. Ensure an appropriate blood pressure cuff size is used.
 Consider the need for referral or urgent care if the patient’s blood pressure is ≥ 180/110 mmHg and there are signs of end organ damage (malignant hypertension), e.g. abnormalities on ECG, or if the patient is pregnant. For further information, see: “Investigate for end organ damage and co-morbidities”
Consider the need for referral or urgent care if the patient’s blood pressure is ≥ 180/110 mmHg and there are signs of end organ damage (malignant hypertension), e.g. abnormalities on ECG, or if the patient is pregnant. For further information, see: “Investigate for end organ damage and co-morbidities”
Consider an out-of-clinic blood pressure assessment
Even when standardised methods for blood pressure measurement are used, clinic readings do not always reflect true blood pressure due to patient-specific psychological, physiological and behavioural factors.5 On average, measurements are 5 – 10 mmHg higher in this setting compared with at-home or ambulatory monitoring.5 Therefore, 24-hour ambulatory monitoring (the “gold standard”) or at-home monitoring should be considered if resources are available to confirm a diagnosis of hypertension and to rule out:
- White-coat hypertension if measurements are consistently elevated despite the absence of obvious risk factors
- Masked hypertension if clinic blood pressure is consistently normal but there are clinical features consistent with hypertension, e.g. signs of end organ damage
For further information on 24-hour ambulatory or at-home monitoring, see: “Out-of-clinic blood pressure testing in primary care” at bpac.org.nz/BPJ/2016/May/blood-pressure.aspx
2. Perform a CVD risk assessment
CVD risk assessment forms the basis for discussions about prognosis and treatment options with the patient and provides information about other factors affecting cardiovascular management, e.g. diabetes, chronic kidney disease (CKD) and the prevention of myocardial infarction and stroke.
A five-year CVD risk assessment should be performed in all patients with a blood pressure persistently ≥ 130/80 mmHg (see: “When to initiate antihypertensive medicines”). In addition, five-year CVD risk assessments are recommended from age 45 years in males and 55 years in females. However, it is recommended that assessments should be:6
- 10 years earlier for people with personal or family history risk factors for CVD or diabetes
- 15 years earlier for people of Māori, Pacific or South-Asian ethnicity
- 20 years earlier for people with severe mental illness
- From diagnosis for people with type 1 or 2 diabetes
The five-year CVD risk thresholds have been established based on the New Zealand primary prevention equations (derived from the PREDICT study), which incorporate a wider range of variables.6 Most patient management systems will have an integrated CVD risk assessment tool. Alternatively, an online CVD risk calculator, with the option of using PREDICT data, is available from: cvdcalculator.com. These tools also provide a pictorial representation of the potential benefits and harms with and without intervention to inform shared decision making with the patient if antihypertensive treatment is ultimately indicated (see: “When to initiate antihypertensive medicines”).
If you do not have an integrated CVD risk assessment module installed in your practice management system (PMS), contact BPAC Clinical Solutions (bpacsolutions.co.nz/contact/) for information about their Primary Care Suite which includes a CVD risk assessment tool. This tool can be accessed via bestpractice Decision Support in your PMS and pre-populates relevant data from patient records to improve useability.
Standard CVD risk calculators may not accurately reflect risk for all groups.
Comprehensive clinical assessment in patients with newly identified hypertension should include evaluation of urine albumin:creatinine ratio (ACR) and eGFR as kidney dysfunction is strongly related to increased CVD risk (see: “A focus on cardiovascular-kidney-metabolic risk” and “3. Investigate for end organ damage and co-morbidities”). CVD risk calculators tailored for use in patients with diabetes often include these parameters, however, others intended for more general use (i.e. irrespective of diabetic status) typically do not, and therefore potentially underestimate CVD risk.
To access the New Zealand Society for the Study of Diabetes (NZSSD) CVD risk calculators for use in patients with diabetes, see: https://www.nzssd.org.nz/resources/section/calculators
A focus on cardiovascular-kidney-metabolic risk
There is substantial interconnectedness between the cardiovascular system, metabolic risk factors (e.g. diabetes, obesity) and CKD. The term "cardiovascular-kidney-metabolic (CKM) syndrome” is being increasingly discussed in the literature. CKM describes a systemic disorder defined by pathophysiological interactions among these three factors, resulting in multi-organ dysfunction/damage and adverse CVD outcomes.a,b
The kidneys have a key physiological role in regulating blood pressure.c Damaged kidneys are less capable of controlling blood pressure, and hypertension is in turn a leading cause of CKD progression.c Albuminuria (or proteinuria) is often the first sign of vascular endothelial dysfunction, typically being present long before there is any clinical evidence of end organ damage such as left ventricular hypertrophy.b An elevated urine ACR is common in a setting of hypertension; one in two newly diagnosed patients have evidence of microalbuminuria (ACR 3 – 30 mg/mmol)d and one in five have evidence of macroalbuminuria (ACR > 30 mg/mmol)e. Elevated urine ACR is strongly associated with an increased risk of CVD and death.e,f
However, classical CVD risk calculators often do not incorporate albuminuria in the assessment and as a result likely underestimate CVD risk in patients with underlying kidney dysfunction. Urine ACR testing is routinely undertaken in patients with diabetes, but evidence suggests it is underutilised in patients without diabetes in New Zealand primary care, even in the setting of known CKD risk (e.g. hypertension, Māori or Pacific peoples).g Urinalysis including ACR testing and eGFR assessment should therefore be considered an essential component of continued CVD risk assessment in patients with newly diagnosed hypertension (see: “3. Investigate for end organ damage and co-morbidities”). If a patient has a low calculated CVD risk (i.e. five year CVD risk < 5%), but has evidence of renal impairment or proteinuria, antihypertensive treatment should still be considered (see: https://bpac.org.nz/2022/ckd.aspx).f ACE inhibitors and ARBs at their maximum tolerated dose should be prioritised initially in these patients to optimise their antiproteinuric effect, as they have the strongest evidence for delaying CKD progression and reducing CVD risk (Table 3).f This differs somewhat to the approach for managing uncomplicated hypertension in international guidelines, where there is increasing emphasis on early use of low-dose dual antihypertensive treatment (i.e. avoiding early high dose monotherapy). However, in patients with established CKD, multiple medicines are often required to achieve blood pressure targets, and this need increases as a patient’s eGFR declines.
For further information on blood pressure management in patients with CKD, see: https://bpac.org.nz/2022/ckd.aspx
References
- Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-kidney-metabolic health: a presidential advisory From the American Heart Association. Circulation 2023;148:1606–35. doi:10.1161/CIR.0000000000001184
- Marassi M, Fadini GP. The cardio-renal-metabolic connection: a review of the evidence. Cardiovasc Diabetol 2023;22:195. doi:10.1186/s12933-023-01937-x
- Ameer OZ. Hypertension in chronic kidney disease: What lies behind the scene. Front Pharmacol 2022;13:949260. doi:10.3389/fphar.2022.949260
- Poudel B, Yadav B, Raut K, et al. Prevalence and association of microalbuminuria in essential hypertensive patients. North Am J Med Sci 2012;4:331. https://pubmed.ncbi.nlm.nih.gov/22912940/
- Shin J-I, Chang AR, Grams ME, et al. Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension 2021;78:1042–52. doi:10.1161/HYPERTENSIONAHA.121.17323
- Mancia G, Kreutz R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens 2023;Publish Ahead of Print. doi:10.1097/HJH.0000000000003480
- Lloyd H, Li G, Tomlin A, et al. Prevalence and risk factors for chronic kidney disease in primary health care in the southern region of New Zealand. Nephrology 2019;24:308–15. doi:10.1111/nep.13395
3. Investigate for end organ damage and co-morbidities
After confirming elevated blood pressure and assessing the patient’s five-year CVD risk, further investigations should include (at a minimum):1, 5, 9
- Dipstick urine test for blood or protein
- Quantification of urinary protein, e.g. by assessing ACR
- Laboratory investigations for electrolytes and creatinine (eGFR), lipids, HbA1c
- An ECG to assess for signs of left ventricular hypertrophy, atrial fibrillation or evidence of historical ischaemic heart disease. Consider referring for an echocardiogram if indicated.
Assess for other symptoms indicative of end organ damage, e.g. chest pain, breathlessness, visual disturbances, transient focal weakness.
Consider ophthalmoscopic examination of the fundus, particularly in patients reporting visual disturbances, as persistently elevated blood pressure can cause retinopathy, choroidopathy and optic neuropathy.4 Key features include copper (or silver) wiring, arteriovenous nicking and retinal haemorrhages.10
For further information on hypertensive retinopathy and example images, see: www.msdmanuals.com/professional/eye-disorders/retinal-disorders/hypertensive-retinopathy
4. Consider secondary causes of hypertension
Most patients diagnosed with hypertension have primary (or essential) hypertension, where there is no clinically identifiable cause.11 While the pathophysiology of primary hypertension is not fully understood, it is thought to involve a complex interplay of genetic predisposition, environmental factors and age-associated stiffening of blood vessels.2
In approximately one in ten patients with hypertension, increased blood pressure measurements are associated with an underlying condition or stressor (secondary hypertension).2 If these factors can be more effectively managed or resolved, it may prevent unnecessary pharmacological intervention. Secondary causes of hypertension should be suspected in young adults (e.g. aged < 30 years) without a family history of hypertension or other risk factors.
Secondary causes of hypertension include: 5, 6, 9
- High alcohol intake, e.g. consistently having > 10 standard drinks per week for females or > 15 standard drinks for males
- Illicit drugs, e.g. amphetamine or cocaine use
- Certain medicines, e.g. oral contraceptives and corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), ciclosporin and decongestants, e.g. phenylephrine
- Obstructive sleep apnoea
- Aortic coarctation, suggested by a diminished or delayed femoral pulse and low or unobtainable blood pressure in the legs, or abnormal differences in the upper and lower extremity arterial pulses
- Renovascular or primary renal disease
- Renal parenchymal disease, including glomerulonephritis, suggested by a history of urinary tract infection or obstruction, haematuria, analgesic misuse or a family history of polycystic kidney disease
- Endocrine disorders, e.g. Cushing’s syndrome (excessive cortisol production), Conn’s syndrome (also known as hyperaldosteronism, involving excessive aldosterone production), phaeochromocytoma (a rare adrenal gland tumour), hypo-/hyperthyroidism
Lifestyle modifications are important for all patients
Healthy lifestyle advice should be given to all patients with persistently elevated blood pressure measurements and reinforced if a diagnosis of hypertension is ultimately made. There is clear evidence from clinical trials that lifestyle changes can reduce mean blood pressure measurements and even prevent hypertension if they are sustained.2
Key modifications to make where applicable include:2, 4, 5, 12
The effectiveness of recommending “lifestyle changes” to control blood pressure varies between patients in primary care. Long-term behavioural modifications can be difficult to make and even harder to maintain. For some patients, despite their best efforts, lifestyle changes will be insufficient to make a clinically significant difference to their blood pressure, but they remain an important aspect of hypertension management even if antihypertensives are introduced.
When to initiate antihypertensive medicines
Patients with a blood pressure of ≥ 160/100 mmHg should be initiated on antihypertensive treatment immediately, in addition to lifestyle changes, regardless of their five-year CVD risk.6
For all other patients with a blood pressure persistently ≥ 130/80 mmHg, the 2018 Ministry of Health cardiovascular risk consensus statement recommends calculating their five-year CVD risk to guide antihypertensive medicine decisions. In patients with:6
- Five-year CVD risk < 5%: antihypertensive treatment is not recommended; proceed with lifestyle changes
- Five-year CVD risk 5 – 15%: consider antihypertensive treatment if blood pressure is ≥ 140/90 mmHg, in addition to lifestyle changes
- Five-year CVD risk ≥ 15%: antihypertensive treatment is recommended, in addition to lifestyle changes
For further information see: “Cardiovascular disease risk assessment and management for primary care” (available at: www.health.govt.nz/publication/cardiovascular-disease-risk-assessment-and-management-primary-care)
Choosing a suitable antihypertensive
All first-line antihypertensives have a comparable blood pressure lowering effect and therefore all are suitable choices for patients without complicating factors or interacting medicines (see: “Consider patient co-morbidities and characteristics”).2, 5
In New Zealand, funded first-line options include (see Table 1 for dosing recommendations):12, 13
- Angiotensin-converting-enzyme (ACE) inhibitors
- Angiotensin-II receptor blockers (ARBs). N.B. Despite previously being regarded as a second-line option after ACE inhibitors, ARBs are now considered to provide comparable benefit for treating patients with hypertension and are often better tolerated.
- Calcium channel blockers
- Thiazide and thiazide-like diuretics
Practice point: ACE inhibitors and ARBs should generally not be prescribed in combination unless under specialist supervision primarily due to the increased risk of renal impairment and hyperkalaemia. Concurrent use is broadly contraindicated in patients with renal impairment or diabetic nephropathy. However, there is some evidence that dual ACE inhibitor/ARB treatment is effective for preventing end-stage kidney disease.
Treatment can be initiated with a single antihypertensive or with two antihypertensives together at low doses (dual antihypertensive treatment; see: “Initial use of two low-dose antihypertensives can be a good starting point for many patients”). Antihypertensive treatment generally takes four-to-six weeks to reach maximum effect.9
For further information on ACE inhibitors, see “Prescribing ACE inhibitors: time to reconsider old habits” at: bpac.org.nz/2021/ace.aspx
Table 1. Recommended adult dosing for commonly used antihypertensive medicines in New Zealand.13 N.B. Lower initial dosing of ACE inhibitors/ARBs (e.g. half the initial dose) and more gradual titration may be required for elderly or frail patients, or in patients with renal impairment, cardiac decompensation, volume depletion or concomitant diuretic use. For further information on dosing recommendations, refer to the New Zealand Formulary: www.nzf.org.nz/nzf_1168.
| Class |
Funded option as of May, 2024 |
Initial dose
(may be lower in some patients) |
Dose range |
| ACE inhibitors* |
Enalapril |
5 mg, once daily |
Usual maintenance dose 20 mg, once daily; maximum 40 mg, once daily |
| Lisinopril |
10 mg, once daily |
Usual maintenance dose 20 mg, once daily;
maximum 80 mg, once daily |
| Perindopril |
4 mg, once daily in the morning |
Maximum 8 mg, once daily |
| Quinapril |
10 mg, once daily |
Usual maintenance dose 20 – 40 mg, daily in 1 – 2 divided doses; maximum 80 mg, daily |
| Ramipril |
2.5 mg, once daily |
Maximum 10 mg, once daily |
| ARBs |
Candesartan |
8 mg, once daily |
Usual maintenance dose 8 mg, once daily; maximum 32 mg, once daily |
| Losartan |
50 mg, once daily |
Usual maintenance dose 50 mg, once daily; maximum 100 mg, once daily |
| Calcium channel blockers |
Amlodipine |
5 mg, once daily |
Maximum 10 mg, once daily |
| Diltiazem
(modified release) |
180 – 240 mg, once daily |
Usual maintenance dose 240 – 360 mg, once daily |
| Felodipine (modified release) |
5 mg, once daily in the morning |
Usual maintenance dose 5 – 10 mg, once daily |
Verapamil
(modified release) |
120 – 240 mg, once daily |
Maximum 240 mg, twice daily |
| Thiazide and thiazide-like diuretics |
Bendroflumethiazide |
2.5 mg, once daily in the morning |
Maximum doses of 10 mg, daily have been used; however, doses higher than 2.5 mg daily, increase the risk of adverse effects and have a limited additional blood pressure lowering effect |
| Chlortalidone |
12.5 – 25 mg, once daily in the morning |
|
| Indapamide |
2.5 mg, once daily in the morning |
|
| Fixed-dose combination treatment† |
Losartan + hydrochlorothiazide |
1 tablet (50 mg losartan + 12.5 mg hydrochlorothiazide), once daily |
Maximum 2 tablets, once daily |
*Cilazapril is another ACE inhibitor that was previously used extensively in New Zealand, however, it is no longer funded for new patients.
†Quinapril with hydrochlorothiazide is another fixed-dose combination treatment that was used in New Zealand, however, it is no longer available due to a stock impurity issue. For further information, see: “Warning: the fixed-dose combination antihypertensive Accuretic (quinapril with hydrochlorothiazide) should no longer be used”.
 Warning: the fixed-dose combination antihypertensive Accuretic (quinapril with hydrochlorothiazide) should no longer be used.
Warning: the fixed-dose combination antihypertensive Accuretic (quinapril with hydrochlorothiazide) should no longer be used.
Read more
Voluntary recalls of the combination antihypertensive Accuretic (quinapril with hydrochlorothiazide) have been occurring worldwide since March, 2022, after the supplier (Pfizer) advised that batches had been contaminated with nitrosamines.14 Long-term exposure to nitrosamines has been associated with a small cumulative risk of cancer.15
PHARMAC has advised that Accuretic will be delisted in early 2023 (N.B. Pfizer has provided no certain timeline as to when a replacement supply without impurities will be available). No new patients should be initiated on Accuretic, and those currently taking it should be switched to an alternative treatment.14 Given that no alternative brand to Accuretic is available in New Zealand, patients who require a fixed-dose combination treatment may be changed to losartan with hydrochlorothiazide.13, 14 Another option is concurrent use of any ACE inhibitor (e.g. quinapril or another choice) or ARB and a thiazide or thiazide-like diuretic (Table 1).13 There are no funded brands of medicine with hydrochlorothiazide as the only active ingredient in New Zealand; suitable alternatives include bendroflumethiazide, chlortalidone or indapamide (for further information, see: nzf.org.nz/nzf_998).
Beta-blockers are no longer considered a first-line antihypertensive unless there is a clinical need
Beta-blockers are less effective at reducing stroke risk compared with other antihypertensive medicines and are often poorly tolerated.5 However, beta-blockers may be preferred early in treatment for patients with some co-morbidities, such as ischaemic heart disease (as they also help to decrease heart rate, increase diastolic filling time, decrease cardiac contractility and reduce myocardial oxygen demand) or atrial fibrillation (as they help to control heart rate).5
Consider patient co-morbidities and characteristics
Two-thirds of patients with hypertension have a co-morbidity, which may in turn influence the suitability of the antihypertensive used (Table 2).4, 5, 16 For example, in patients with hypertension and gout, losartan is often favoured due to its dual blood pressure lowering/uricosuric effects, while thiazide diuretics should be avoided as they promote urate reabsorption in proximal renal tubules.4, 5
Other patient characteristics may also affect the choice of antihypertensive. For example, ACE inhibitors or ARBs are sometimes favoured in patients aged under 55 years due to the potential for a more significant reduction in renin levels.1, 17 However, exposure to ACE inhibitors and ARBs increases the risk of fetal abnormalities when used during pregnancy.18 Therefore, any females planning or considering pregnancy should ideally use an alternative medicine, or switch from an ACE inhibitor/ARB to another option once the pregnancy is confirmed.18 Recommended antihypertensives during pregnancy include labetalol, nifedipine and methyldopa.18
Table 2. Antihypertensive recommendations based on patient co-morbidities and characteristics.1, 4, 5, 16
| Co-morbidity or characteristic |
Potentially Beneficial |
Avoid |
| Chronic kidney disease |
- Prioritise an ACE inhibitor or ARB at the maximum tolerated dose
- Calcium channel blockers
- Loop diuretics (if eGFR < 30 mL/min/1.73 m2)
|
- High salt intake
- Thiazides in patients with more than mild renal impairment
|
| Diabetes (Type 1 and Type 2) |
- Prioritise an ACE inhibitor or ARB
- Thiazide (or thiazide-like) diuretic
- Calcium channel blocker
|
- Beta-blockers
- High dose thiazide diuretics (low doses are acceptable)
|
| Heart failure or asymptomatic left ventricular dysfunction |
- ACE inhibitors, ARBs or an angiotensin receptor-neprilysin inhibitor (ARNI) are first-line
- Other medicines used in the treatment of heart failure also have BP-lowering effects, e.g. diuretics, beta-blockers (dose adjustments may be required for patients with renal dysfunction), SGLT-2 inhibitors and mineralocorticoid receptor antagonists
|
- Non-dihydropyridine calcium channel blockers (e.g. diltiazem, verapamil)
- Beta-blockers in patients with uncontrolled heart failure
|
| Acute myocardial infarction |
- Beta-blockers without intrinsic sympathomimetic activity, e.g. carvedilol
- ACE inhibitors or ARBs
|
|
| Atrial fibrillation |
- Beta-blockers
- Rate limiting calcium channel blocker, e.g. diltiazem
- ACE inhibitors or ARBs
|
|
| Angina |
- Beta-blockers
- Calcium channel blockers
- ACE inhibitors or ARBs
|
|
| Cerebrovascular disease, i.e. stroke |
- ACE inhibitors or ARBs
- Calcium channel blockers
- Low-dose thiazide diuretics
|
- Beta-blockers
- Thiazide diuretics in very elderly patients or those with poor daily fluid intake as they could contribute to hypoperfusion
|
| Asthma/COPD |
- No specific recommendations
|
- Beta-blockers, however, low-dose bisoprolol (or metoprolol) can be used if required in patients with asthma/COPD and heart failure
|
| Gout |
- Losartan (has a uricosuric effect)
|
|
| Pregnancy |
- Labetalol
- Nifedipine
- Methyldopa
|
|
Initial use of two low-dose antihypertensives can be a good starting point for many patients
The blood pressure lowering effect of any single antihypertensive at its optimal dose has been estimated to be < 10 mmHg on average.19 Therefore, initial monotherapy is unlikely to be effective in many patients with hypertension (despite previously being standard practice) and multiple medicines are frequently required to achieve treatment targets. As such, international guidelines now recommend a more pragmatic approach to treatment, with initial use of two low-dose antihypertensives together (dual antihypertensive treatment) being indicated in many cases (Figure 2).5 For further information, see “A closer look at the evidence supporting initial treatment with two low-dose antihypertensives”. While selection may be influenced by patient co-morbidities (Table 2), an ACE inhibitor or ARB with a dihydropyridine calcium channel blocker such as amlodipine has been demonstrated to be the most effective combination based on evidence from the ACCOMPLISH trial.20 Single antihypertensive use may still be a suitable starting point for some, e.g. elderly patients or those already close to their blood pressure target.
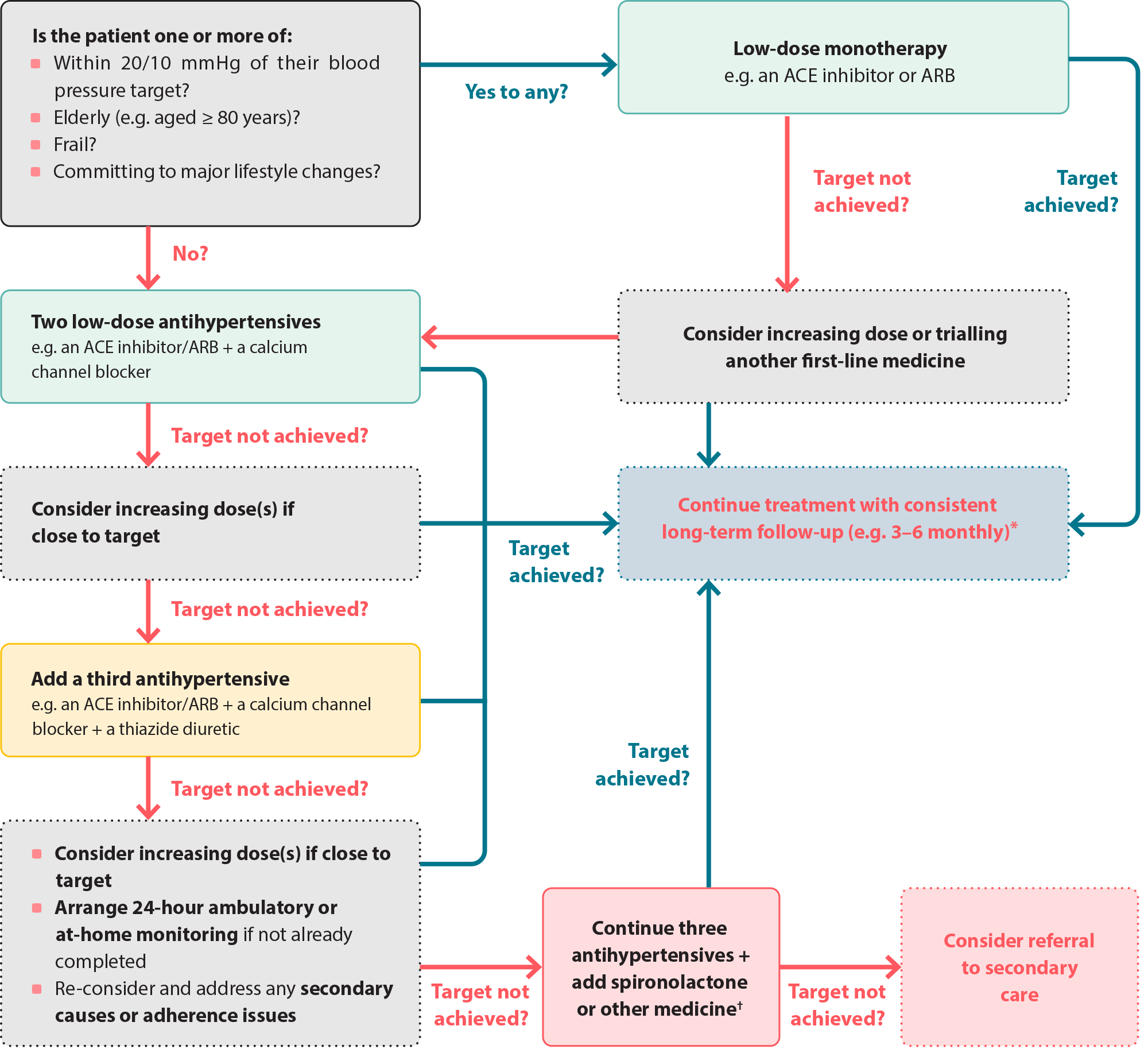
Figure 2. A pragmatic approach to antihypertensive treatment for patients with hypertension.5
* Blood pressure should be monitored at least every four-to-six weeks during medicine titration until blood pressure targets have been achieved. The frequency of follow-up in the long-term depends on a range of patient-specific factors, e.g. the severity of hypertension in the context of the patient’s overall CVD risk.4
†Such as a beta-blocker or an alpha-blocker
A closer look at the evidence supporting initial treatment with two low-dose antihypertensives
Half the standard dose of any first-line antihypertensive still provides approximately 80% of the maximal blood pressure lowering effect.19 In addition, two low-dose antihypertensives used together are approximately five times more effective at lowering blood pressure than doubling the dose of a single antihypertensive (Figure 3).21 Given that hypertension is almost always caused by a combination of pathophysiological processes, countering it using medicines with different mechanisms of action is therefore thought to improve treatment efficacy.22 Safety is sometimes cited as a concern when prescribing two low dose antihypertensives together, however, the risk of adverse effects is often actually greater with a high dose of a single antihypertensive.23
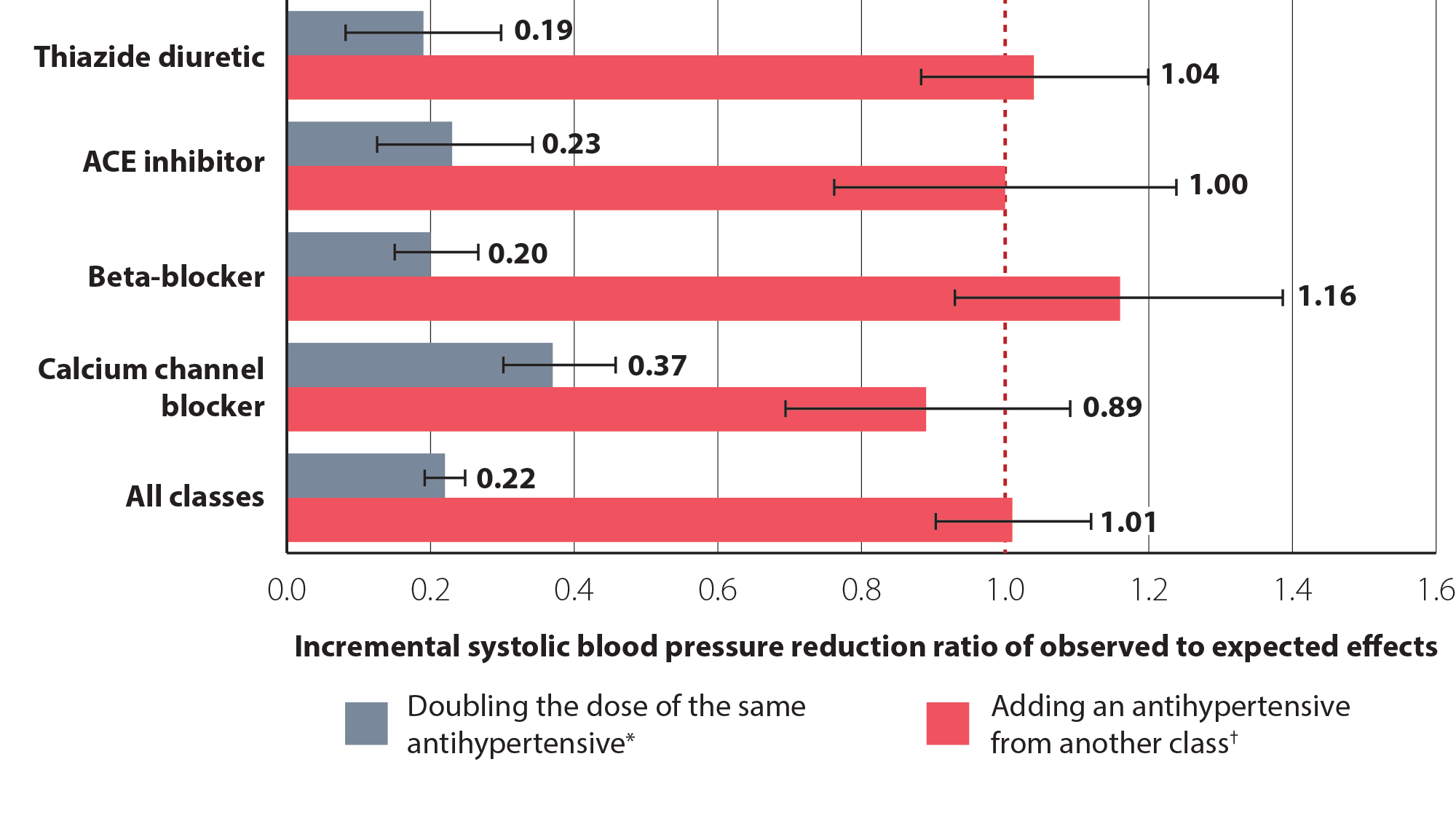
Figure 3. Two low-dose antihypertensives used together have a greater
blood pressure-lowering effect than doubling the dose of one antihypertensive.21
*From a standard initial dose to twice the standard initial dose
†Both antihypertensives at the standard initial dose
N.B. An incremental effect of 1.0 indicates that the systolic blood pressure-lowering effect is exactly additive, 0.5 indicates a sub-additive effect (equivalent to 50% of the additive effect). Figure adapted from Wald DS, et al., 2009.21
Numerous clinical trials have addressed the concept of the “optimal” blood pressure target for patients with hypertension, and there are discrepancies in recommendations between different international guidelines. In 2018, New Zealand Ministry of Health guidelines specified a target clinic blood pressure of < 130/80 mmHg for most people.6 However, there has been a shift in some international guidelines to instead use individualised blood pressure targets based on the patient’s CVD risk, co-morbidities and treatment objectives (Table 3).4, 5 There is strong evidence supporting a lower clinic blood pressure target (i.e. < 130/80 mmHg) for patients with a high CVD risk.4 However, the absolute benefits are likely outweighed by the potential harms in those with a lower CVD risk, and therefore a more conservative target is often more appropriate for this group (i.e. < 140 mmHg).4, 24
See “Update: European Society of Hypertension (ESH) 2023 guidelines now available on the management of arterial hypertension” for further discussion about blood pressure targets
Intensive blood pressure management
The potential benefit of using a stricter blood pressure target (systolic blood pressure < 120 mmHg) in patients with high CVD risk has been investigated in large multi-centre clinical trials such as SPRINT and ACCORD.25, 26 These studies have yielded mixed results, and there is debate as to whether any evidence of benefit outweighs the potential harms in real-world populations, particularly when strict eligibility criteria is used in randomised controlled trials.27 In addition, it remains uncertain whether intensive blood pressure lowering has a uniform effect and tolerance across all age groups.27 Findings from the 2021 Chinese STEP study indicate that intensive blood pressure management may be beneficial in some older patients, however, it is uncertain how applicable these findings are to Western populations which have different patterns of CVD and lifestyle factors.28
There are currently no recommendations for applying intensive targets in primary care. If this decision is being considered for any reason, it should realistically take into consideration other CVD risk factors the patient may have, their co-morbidities, family history, concurrent medicines and overall health; the relative importance of each should be determined by the patient’s preference and the clinician’s expertise.
For further discussion on intensive blood pressure management, see “Go low or no? Managing blood pressure in primary care” at: bpac.org.nz/2017/blood-pressure.aspx
Table 3. Recommendations for individualising patient blood pressure targets.4, 5
|
Clinic measurement |
24-hour ambulatory or at-home measurement |
“High” CVD risk
including current atherosclerotic CVD, heart failure, reduced ejection fraction, diabetes, CKD, aged ≥ 65 years, five-year CVD risk of ≥ 15% |
< 130/80 mmHg |
< 125/80 mmHg |
“Lower” CVD risk
None of the above risk factors |
< 140/90 mmHg |
< 135/90 mmHg |
| Frailty, dementia, limited life expectancy |
Discuss treatment goals to guide decision making; targets can be more lenient and antihypertensive doses may need to be reduced or stopped entirely depending on patient-specific factors. |
Age alone is not a reason to dial back treatment
The risk of falls or orthostatic hypotension are often cited as concerns when considering blood pressure management in elderly patients (e.g. those aged ≥ 80 years), however, there is no reason to withhold antihypertensives in anyone based on age alone.4, 29 Routine treatment should generally be offered to elderly patients for as long as they wish to take it.4 It is their “biological age”, rather than their “chronological age”, that may be a reason for avoiding use based on individual treatment goals. Nevertheless, blood pressure management is one of the few interventions that reduces mortality risk in frail, elderly people.4
Read the evidence
The 2008 Hypertension in the Very Elderly trial (HYVET) treated patients aged over 80 years with hypertension averaging 173/91 mmHg for an average of 1.8 years with indapamide and perindopril to a blood pressure target less than 150/80 mmHg.30 Stroke rates were reduced by 30%, heart failure mortality by 64% and all-cause mortality by 21%. Despite this study being published almost 15 years ago, it is one of the few randomised controlled trials that specifically investigates the benefits of blood pressure management in people aged > 80 years (most exclude such patients).
If antihypertensive treatment is initiated in an elderly patient, starting with low-dose monotherapy is generally recommended, and this approach may be more tolerable as gradually reducing blood pressure is less likely to cause adverse effects, e.g. postural hypotension.29 Close monitoring and more lenient targets are also appropriate, and the treatment intensity should be reduced if there are any concerning emerging features, e.g. cognitive impairment.29 If an elderly patient is already taking multiple antihypertensives and becomes progressively more frail over time, it may be suitable to reconsider the treatment intensity depending on treatment priorities, e.g. lower doses or de-escalating treatment to monotherapy.
Poor medicine adherence is a significant barrier to achieving targets
Poor adherence to antihypertensive treatment is a common issue for many patients with hypertension and is an indicator of poor prognosis.5 Reasons for non-adherence are often multi-faceted and can be difficult to address in primary care.
Potential strategies to encourage and improve adherence include:5
- Educating patients at the time of the initial prescription about their medicine(s) and how it works; this message should be reinforced at each appointment. In particular, if a decision is made to initially prescribe two low dose antihypertensives together, emphasise why it is important that both are taken consistently, i.e. to maximise blood pressure control while reducing the risk of adverse effects (compared with high dose monotherapy).
- Selecting antihypertensives with once daily dosing
- Reducing polypharmacy, e.g. using a fixed-dose combination, if available
- Using pill boxes, blister packaging or electronic reminders (e.g. smartphone apps)
- If adverse effects are problematic, consider night-time dosing, which can limit the perception of adverse effects. Further benefits for night-time dosing have been suggested, however, the evidence at present is inconsistent (see: “Night-time antihypertensive dosing: does it reduce the risk of CVD events?”).
Patients starting antihypertensive treatment should initially be reviewed at least every four-to-six weeks to assess the efficacy of their regimen.9 Shorter review times may be considered for some patients, e.g. every two weeks if the patient’s measurement was significantly elevated at baseline. If a target is not achieved, the next step depends on how well the patient tolerates treatment and how close they are to their objective (Figure 2):5
- If the patient is close to their target blood pressure and the antihypertensive medicine(s) are well tolerated: increase the dose of their existing antihypertensive(s) and re-emphasise the importance of lifestyle changes
- If the patient is not close to their target blood pressure and adherence is not an issue: add an additional antihypertensive and re-emphasise the importance of lifestyle changes, e.g. for patients who started with two low dose antihypertensives such as an ACE inhibitor (or ARB) and a calcium channel blocker, add a thiazide diuretic
Resistant hypertension
If a patient’s clinic blood pressure remains > 140/90 mmHg after treatment with an ACE inhibitor or an ARB, plus a calcium channel blocker and a thiazide diuretic at their optimal (or maximally tolerated) dose, then their hypertension is considered “resistant”.5 Patient adherence to treatment and possible secondary causes should be re-examined, and an added emphasis placed on lifestyle changes.5 In addition, if the patient is taking optimal, or maximum tolerated, doses of antihypertensive medicines, an appropriate specialist opinion is recommended, if this has not been sought already.5
Ultimately, an additional antihypertensive medicine may be required. Options to consider include:5, 9, 13
- Spironolactone – appropriate in combination with another diuretic if serum potassium is ≤ 4.4 mmol/L and eGFR is ≥ 45 mL/min/1.73m2. Spironolactone may be particularly beneficial as many patients with resistant hypertension have high aldosterone levels (even if they do not have clinically apparent primary hyperaldosteronism); spironolactone impairs aldosterone binding to mineralocorticoid receptors which limits vascular resistance. N.B. Spironolactone should be introduced cautiously with ongoing monitoring of serum potassium and creatinine.
- Beta-blocker – no longer considered a first-line antihypertensive for patients with uncomplicated hypertension but may be useful if indicated for a co-morbidity, e.g. atrial fibrillation. All beta-blockers have comparable blood pressure lowering effects.
- Alpha-blocker (e.g. doxazosin) – beneficial for lowering blood pressure but should be used with caution in females as these medicines may sometimes cause urinary stress incontinence and loss of bladder control. A scenario where alpha-blockers may be considered is in males with hypertension who also have benign prostatic hyperplasia to alleviate nocturia.
Novel techniques for treating patients with resistant hypertension
A range of novel procedure- and device-based strategies have been investigated for the management of resistant hypertension. For example, renal sympathetic nerve denervation is a catheter-based technique used to ablate specific portions of renal artery nerves, thereby limiting sympathetic activity and the associated effects on blood pressure.35 Despite promising results in clinical trials,36 there is currently insufficient evidence to support routine use of this and other novel treatments in patients with resistant hypertension.4 Patients who may potentially benefit from such techniques (e.g. those with hypertension resistant to all suitable pharmacological treatment options) should be discussed with a cardiologist.
Once a blood pressure target has been achieved, continued long-term follow-up is important to ensure levels are maintained, and to reinforce the importance of medicine adherence and a healthy lifestyle. Guidelines suggest patients with hypertension should be reviewed three-to-six-monthly.5, 9 However, less frequent review may be more practical for some patients with stable blood pressure measurements, e.g. annually in otherwise healthy patients who are also committing to lifestyle changes and take their medicines regularly. These appointments also provide an opportunity to monitor electrolytes, renal function and ACR ratio, in addition to checking for any emerging signs of end organ disease and associated conditions, e.g. retinopathy, heart failure.
Night-time antihypertensive dosing: does it reduce the risk of CVD events?
Blood pressure is associated with circadian variability; in many people, blood pressure drops during the night by 10 – 20% (versus daytime measurements), before increasing upon waking in the morning – a phenomenon known as the “morning surge”.31 People with hypertension who lack this morning surge due to persistently elevated nocturnal measurements (“non-dippers”) are more likely to have end organ damage, and have an increased risk of cardiovascular events.31
The 2019 Hygia Chronotherapy trial (N = 19,168) demonstrated that night-time antihypertensive dosing was associated with a lower mean systolic blood pressure while sleeping, and reduced the relative risk of primary CVD outcomes by 45% compared with morning administration.*32 This corresponded to an absolute risk reduction of 5.4% for night-time versus morning antihypertensive dosing over the median 6.3 years of follow-up.32 Study authors proposed that night-time dosing of antihypertensives enhanced their effects through “circadian rhythm-dependent influences both on their pharmacokinetics and pharmacodynamics as well as on the mechanisms of blood pressure regulation”.32
While the Hygia trial findings appear promising at first glance, further investigation is required before night-time dosing is routinely recommended in primary care for the sole purpose of greater CVD protection. Since being published, concerns have been raised around the trial design, conduct and the potential mechanism of effect.33 In addition, the question “why would an antihypertensive work better depending on the time of day it is taken once it reaches a steady state?”† remains unaddressed.33
In October, 2022, findings released from the prospective multi-centre TIME trial (N = 21,104) indicate that night-time antihypertensive dosing provides no additional protection against myocardial infarction, stroke and vascular death compared with morning dosing over five years.34 No additional harms associated with night-time dosing were reported.34 Therefore, in contrast to the Hygia trial conclusions, these outcomes suggest patients should simply take their antihypertensive medicines at the time of day most convenient for them and which minimises adverse effects.
*Primary CVD outcomes was defined as a composite endpoint of myocardial infarction, coronary revascularisation, heart failure, ischaemic stroke, haemorrhagic stroke and CVD death
†Steady state occurs when rate of drug absorption equals the elimination rate, i.e. the concentration consistently remains within the range expected to achieve a desired treatment effect
New ESH hypertension guidelines have been released, providing clinicians with further direction when treating patients with hypertension. The 2023 update largely reflects the recommendations established in the previous version of the guideline (2018), but has been expanded based on more recent research, and considers a wider range of conditions and practical concepts, e.g. greater emphasis on out-of-office measurements.
As outlined in the 2018 New Zealand Ministry of Health cardiovascular risk consensus statement, the 2023 ESH guidelines note that antihypertensive prescribing decisions should usually be made in accordance with the patients associated CVD risk, not their blood pressure in isolation (unless it is significantly elevated, e.g. ≥ 160/100 mmHg). These guidelines also endorse the use of two low-dose antihypertensives as a starting point for most patients requiring antihypertensive treatment, with a preference for once daily dosing and single fixed-dose combination pills (if available). While any combination of first-line antihypertensives can be prescribed (except for ACE inhibitors with ARBs), it is recommended that treatment “should be preferentially based on combinations of an ACEi or an ARB with CCB or a thiazide/thiazide-like diuretic”.
The place of beta-blockers in treatment is also revisited. While this medicine class is less effective at preventing stroke compared with first-line antihypertensives, beta-blockers are noted as having other favourable effects across the management of at least 50 conditions that may co-exist with hypertension. Therefore, while their positioning in the antihypertensive hierarchy remains unchanged for patients with uncomplicated hypertension, the 2023 ESH guidelines emphasise that from a practical perspective they should still be considered as a monotherapy or at any stage in combination treatment in patients with relevant co-morbidities, e.g. heart failure, atrial fibrillation.
The 2023 ESH update recommends that a blood pressure target of < 140/80 mmHg is appropriate for most patients “because this accounts for the major portion of the protective effect of BP-lowering”. If antihypertensive treatment is well-tolerated, the systolic blood pressure objective can be further reduced to < 130 mmHg in most patients aged < 80 years (ideal range 120–129/70–79 mmHg). Lowering the diastolic target threshold from < 90 mmHg (suggested in 2018 ESH guidelines) to < 80 mmHg was justified based on the incremental reduction in CVD outcomes and mortality observed in RCTs. Intensive blood pressure management (i.e. targeting blood pressure < 120/70 mmHg) continues to be recommended against, mainly because any small protective effect demonstrated in clinical trials is outweighed by the increased risk of harm and discontinuation in the general population.
The information in this update box represents a summary of selected content. To review the full guidelines, including management recommendations for specific demographics and clinical conditions, see: Mancia G, Kreutz R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens 2023;Publish Ahead of Print. doi:10.1097/HJH.0000000000003480
