INR monitoring is essential for all patients treated with warfarin
International Normalised Ratio (INR) testing is well established as an integral part of warfarin treatment. INR has
a critical role in maintaining the warfarin response within a therapeutic range, to provide the benefits of anticoagulation,
while avoiding the risks of haemorrhage (Figure 1).
Therapeutic monitoring of warfarin treatment requires two key elements to be undertaken if it is to be successful: the
measurement of the INR and an interpretation of the result in order to advise on dosage of warfarin and when the next
test should be performed.
INR levels can be difficult to control
Although regular testing of INR levels is essential for all people taking warfarin to maintain control of the INR, in
practice, INR levels show considerable intra-patient variability. A study has demonstrated that a group of stable patients,
on long-term warfarin treatment achieved the therapeutic range for INR approximately 55% of the time.2
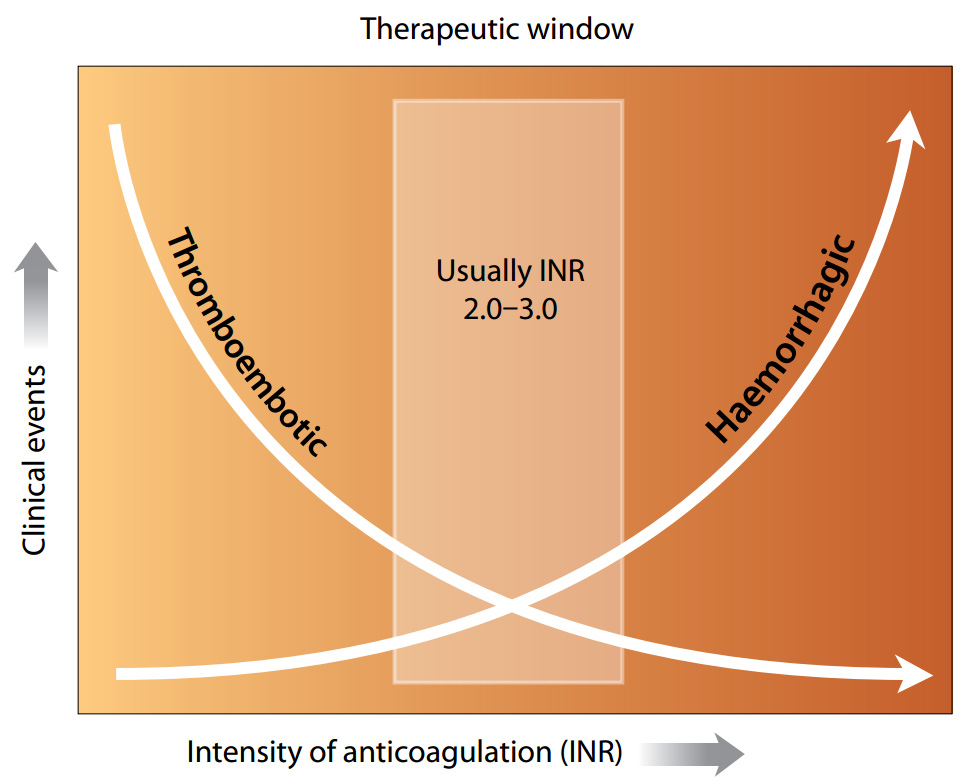
Figure 1: Balancing the risk of anticoagulation treatment (adapted from Blann, 2003)1
Maintaining good systems is important
It is important that practices develop a standardised management protocol for all patients treated with warfarin, in
order to optimise health outcomes, by achieving tighter control.
The plan for anticoagulation therapy should be detailed in the patient’s clinical notes, using a standardised
method, to minimise misunderstandings. The method chosen will depend on how clinical records are managed within the practice
but there should at least be a standard location within the patient notes for the following information:
- A note that the patient is on warfarin
- Condition for which warfarin has been prescribed
- Target INR range
- Planned duration of treatment
- Brand of warfarin
The information that a patient is on warfarin must be immediately obvious to any clinician who accesses the patient’s
clinical record.
Managing warfarin treatment
INR testing schedule
Regular testing of the INR is essential for all people taking warfarin. The risk of bleeding while on warfarin is greatest
in patients who have not previously received warfarin, and in the first three months of treatment.3
Any patient on warfarin should be aware of the risks and early warning signs of bleeding, and they should be followed
closely, during the first three months in particular, to ensure that the INR does not exceed 3.0.
After this time period, the frequency of INR testing can be reduced. For most people once the INR is stable, the rate
of INR testing can be extended to two weekly and then four to six weekly. In some stable patients the frequency may be
extended out to eight weeks.4 However, people with higher levels of risk, e.g. comorbidities, may need more
frequent testing.
Target INR range and duration of treatment
In most situations the INR target is 2.5 (target range 2.0 – 3.0). This range is appropriate for the prophylaxis or
treatment of venous thromboembolism and reduction of the risk of systemic embolism for people with atrial fibrillation
and valvular heart disease.5 In some situations higher ranges are more appropriate. The target INR may vary
depending on individual clinical situations. The target INR for mechanical prosthetic valves is dependent on the type
of valve replacement used.6
The duration of warfarin therapy for a provoked DVT or PE is 13 weeks. For unprovoked DVT or PE the duration again is
13 weeks, but for individual patients within their clinical context, the indefinite use of warfarin may be appropriate.5 For
atrial fibrillation, cardiomyopathy and valvular heart disease (selected cases) an indefinite period of warfarin treatment
is recommended.6
Managing alterations in the INR
Some fluctuations in INR level can be expected, and for minor variations, changes in weekly doses are usually not required.
For more significant fluctuations, use of a standard guide is important to reduce the risk of incorrect dosing. The use
of dosing calendars for more complicated dosage sequencing may be of benefit.
Changes in warfarin dosage may take several days to affect INR level, therefore it is important that doses are not adjusted
more frequently than every four to five days.
Changes in the INR level in a usually stable patient may be due to a number of reasons, including:7,8
- Major changes in diet or alcohol intake
- Drug interactions (pharmaceutical or complementary)
- Systemic or concurrent illness
- Non-adherence to dosage regimen
- Unknown causes
Diet or alcohol
Patients on warfarin are usually advised to consume a reasonably consistent proportion of vitamin K rich foods such
as broccoli, spinach and cabbage. This is probably most relevant in patients who have had markedly reduced food intake
because of illness, hospitalisation, travel and fad diets.9 A recent study suggests that the role of excessive
dietary vitamin K may have been overstated, with the exception of natto (Japanese fermented soybean) which causes a marked
and prolonged inhibition of warfarin.10
Increased consumption of alcohol (particularly binge drinking) can affect warfarin control although moderate, regular
alcohol consumption has little effect.
Drug interactions
Many medicines and herbal products can interact with warfarin. An interaction can occur when the interacting agent is
started or stopped or when the dose is altered. Whilst most interactions involve a change in the INR, it is important
to recognise that some interactions cause an increase in bleeding without alteration of the INR, e.g. NSAIDs, aspirin
and SSRIs (Table 1).
Table 1 shows some of the important interactions with warfarin. It is not all-inclusive and practitioners should always
check if there is a clinically significant interaction if they are prescribing a medicine for a person taking warfarin.
Patients should also be advised not to take any other prescribed medicines, over-the-counter medicines or food supplements/herbal
products without consulting their doctor or pharmacist.
 For a complete list of interactions and advice on managing interactions such
as when to check the INR, refer to appropriate information resources such as a formulary or your PMS system.
For a complete list of interactions and advice on managing interactions such
as when to check the INR, refer to appropriate information resources such as a formulary or your PMS system.
Table 1: Some of the main medicines, medicine classes and other agents that can interact with warfarin
(adapted from Juurlink, 2007)11
| |
Risk of Bleeding |
Mechanism
|
Antibiotics
|
|
|
Most antibiotics but especially macrolides, metronidazole, quinolones and cotrimoxazole
|
 |
Inhibition of vitamin K synthesis by intestinal flora, inhibition of warfarin metabolism
or both |
| Rifampicin* |
 |
Induction of hepatic metabolism
|
| Antifungals |
|
|
Fluconazole, miconazole (including gel and vaginal preparations)
|
 |
Inhibition of warfarin metabolism |
| Antidepressants |
|
|
Serotonergic agents (SSRIs and venlafaxine)
|
 |
Inhibition with platelet function – increased bleeding risk without alteration of INR. Some,
e.g. fluoxetine, paroxetine, can also inhibit warfarin metabolism |
| Antiplatelet agents |
|
|
Aspirin, clopidogrel, dipyridamole
|
 |
Interference with primary haemostasis – increased bleeding risk without alteration of INR |
Amiodarone
|
 |
Inhibition of warfarin metabolism |
| Anti-inflammatory agents |
|
|
NSAIDS, Cox-2 inhibitors
|
 |
Direct mucosal injury, antiplatelet effects may also have a role. Increased bleeding risk without
alteration of INR. Inhibition of warfarin metabolism and an increase in INR rarely reported with some NSAIDs |
| Analgesics |
|
|
| Tramadol |
 |
Inhibition of warfarin metabolism |
| Paracetamol |
 |
Direct interference with vitamin K cycle
Interaction possible with chronic, regular use of paracetamol, short-term (a few days) unlikely to interact
|
| Alternative remedies/foods |
|
|
Ginkgo, fenugreek, chamomile, dong quai, cranberry products
|
 |
Unclear, multiple mechanisms |
| St John’s wort* |
 |
Unclear, possible effects on warfarin metabolism
|
Foods with high vitamin K content, e.g. leafy greens, broccoli*
|
 |
Increased vitamin K synthesis antagonises anticoagulant effect of warfarin |
*These agents will reduce the bleeding risk but the INR may be become sub-therapeutic and warfarin dose
may need to be increased
Notes:
- Interactions do not occur, or are not significant, in everyone. There are many variables including genetic factors.
- This table does not include all possible interactions with warfarin. Please check before prescribing or recommending
any medicine, herbal product or food supplement
Systemic or concurrent disease
Many systemic diseases can influence INR results:
- Congestive heart failure – may cause hepatic congestion of blood flow and inhibit warfarin metabolism, this may be
particularly troublesome during exacerbations of heart failure.
- Hypothyroidism – decreased catabolism of vitamin K clotting factors may decrease INR values.
- Hyperthyroidism – conversely, hyperthyroidism may increase catabolism of vitamin K clotting factors and increase INR
values.
- Liver failure – may cause elevation of INR due to reduced production of clotting factors.
- Other illnesses – other intermittent conditions such as fever, vomiting and diarrhoea may affect the INR; ill patients
may also reduce their usual dietary intake.
Non-adherence to dosage regimen
An erratic INR may reflect non-adherence to the medicine regimen, often due to misunderstandings of dosage requirements.
A missed dose of warfarin is usually reflected in the INR result two to five days after the missed dose,12 although
a response may be seen within 16 hours.13
Unknown causes
In many cases, no explanation may be found for unstable INR values. It may be worthwhile discussing aspects of the dosing
regimen. Changes in the INR may also be the result of occult causes, such as undisclosed drug use, lifestyle and medical
causes.
Computerised decision support
Computerised decision support is a very useful tool for maintaining therapeutic INR levels in patients receiving anticoagulant
treatment. There is evidence that computerised decision support can achieve improved therapeutic control in terms of INR,
when compared with human performance.14
A meta-analysis of randomised controlled trials compared computerised decision support methods of determining warfarin
dosage with traditional manual methods in 3416 patients.15 The computerised decision support groups did better
in terms of percentage of INR tests within target (65% computer group, 59% manual group, NNT 17) and showed a significant
reduction in the incidence of bleeding (2% computer group, 4.4% manual group).
A randomised controlled trial compared the INR control (by the percentage of time within-target) of two groups of patients
attending an anticoagulation clinic in Italy.16 One group were managed using computerised decision support
and the other group were dosed using manual methods by experienced haematologists. The INR control in the computerised
decision support group was significantly better (71% of time within range for the computer group, 68% for manual group)
and fewer tests were needed to achieve this control.15
One of the advantages of computerised decision support tools is that information can be easily retrieved, providing
many opportunities for clinical practice audit, including identifying patients who are on anticoagulant treatment but
are not receiving INR monitoring.
 The bpacnz clinical audit “Safe and effective anticoagulation
with warfarin” has been recently updated and is available to download.
The bpacnz clinical audit “Safe and effective anticoagulation
with warfarin” has been recently updated and is available to download.
Transfer of care across the primary – secondary interface is associated with a high risk
Transfer of care across the primary – secondary interface is associated with a high risk
Transfer of the care of a patient on warfarin treatment from secondary to primary care is associated with a high risk
for several reasons:
- Poor communication on discharge may leave the primary care clinician with inadequate information to make safe testing
and dose adjustment decisions
- Patients may be discharged from hospital with tablet strengths, which were used for loading doses but are inappropriate
for maintenance therapy. Warfarin has a very long half-life, so accumulates, leading to over-anticoagulation
- Patients often leave hospital with other medicines, e.g. antibiotics, which can interact with warfarin
Some New Zealand hospitals have developed protocols for the timely transfer of information about warfarin therapy to
primary care on patient discharge. Essential details have been found to be:
- Condition for which warfarin has been prescribed
- Target INR range
- Planned duration of treatment
- Brand and strength of warfarin tablets given
- Last three doses
- Last three INR levels
- Date next INR test is due
It is useful to also record this information in the patient’s anticoagulation record (“The Red Book”).
New Zealand hospitals use a variety of warfarin initiation protocols and there is little evidence that one is any better
than another. It is recommended to follow on with the protocol initiated in secondary care for patients who start warfarin
in this environment. It would be helpful for primary care clinicians to become familiar with local hospital protocols.