Arranging a syringe driver for a patient
Hospice or district nursing services can provide equipment and certified staff who can work with General Practitioners,
patients and their families/whānau. Many patients will also be under the care of a palliative care physician. It
is essential that there is good communication between the people who are providing care and support for the patient and
their family (this also includes community pharmacy). Many residential aged care facilities have syringe drivers on site
and staff trained in their use.
 Hospice New Zealand offers a training programme on managing syringe drivers
in primary care. For further information see: www.hospice.org.nz
Hospice New Zealand offers a training programme on managing syringe drivers
in primary care. For further information see: www.hospice.org.nz
Most symptoms can be controlled with a continuous subcutaneous infusion
In a palliative care setting, subcutaneous administration of medicines given via a syringe driver is useful for managing
symptoms such as pain, nausea, anxiety and restlessness. Injectable forms of medicines to control symptoms can be given
alone, or mixed together in a syringe depending on their physical and chemical compatibility and the diluents used (see
below).
Choice of medicine and prescribing
In palliative care, medicines may be prescribed for unapproved indications, be administered by an unapproved route or
given in doses not seen in routine day-to-day practice.5 Most medicines can be used in a subcutaneous infusion,
however, chlorpromazine, prochlorperazine and diazepam are contraindicated as they can cause skin reactions at the injection
site.
Infusions for administration via continuous subcutaneous infusion using a syringe driver should be prescribed to run
over 24 hours, although medicines mixed together may be pharmaceutically compatible and stable for longer than this.
The patient should ideally be reviewed every day so that medicine doses can be adjusted according to their needs.
When prescribing consider:
- The patient's medicine requirements for 24 hours
- The doses that may be required for breakthrough symptoms - these need to be available for immediate use
- The choice of diluent
- The compatibility of the medicines required to manage symptoms (Table 2). In general, avoid combining more than three
medicines in one syringe (occasionally more than one syringe driver is required)
Table 2. Compatibility of medicines for syringe driver infusions commonly prescribed in general practice
(Adapted from Palliative Care Handbook 2012).7
Click to image to enlarge
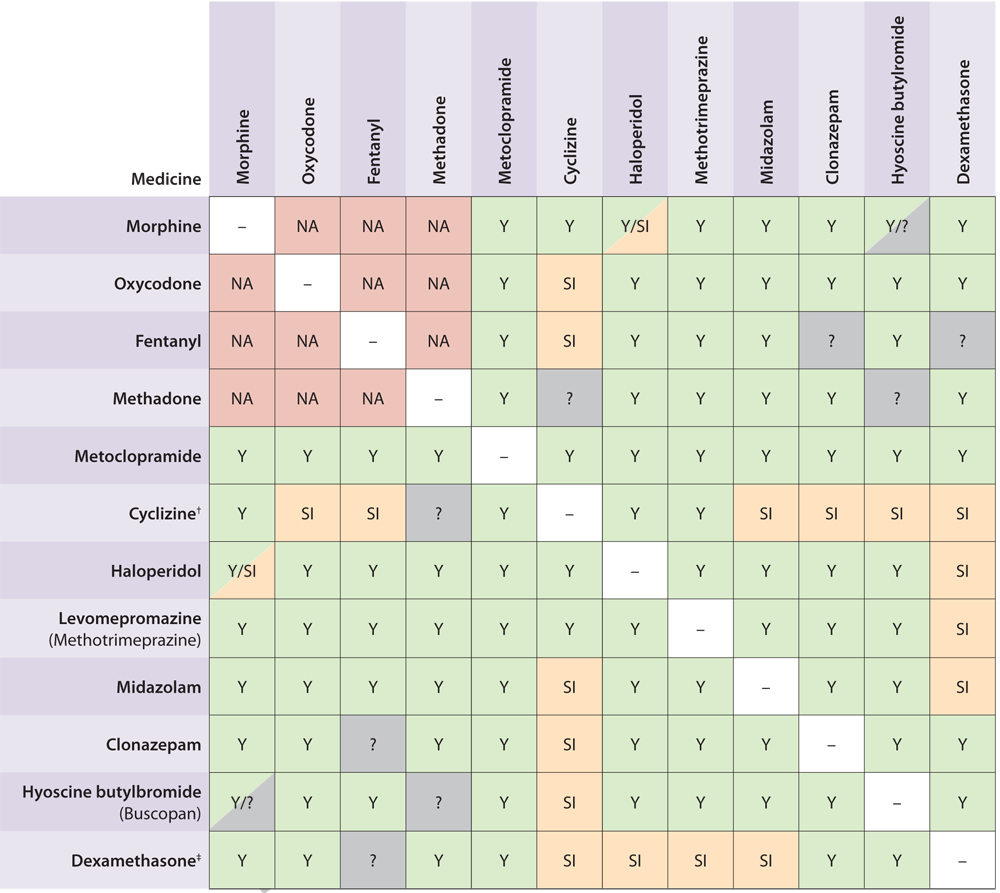
Y = Compatible
SI = Sometimes incompatible (usually at higher doses)
NA = Not usually used together
? = unknown
 If more than two medicines are to be mixed in an infusion, refer to The
Palliative Care Handbook 2012 or contact your local hospice for commonly used combinations and additional compatibility
information
If more than two medicines are to be mixed in an infusion, refer to The
Palliative Care Handbook 2012 or contact your local hospice for commonly used combinations and additional compatibility
information
Choice of diluent
The choice of diluent for the infusion solution varies according to local guidelines as there is evidence for and against
the two most commonly used diluents - sterile water (water for injection) and normal saline (NaCl 0.9%).3 In
general, sterile water is used.
Sterile water is compatible with most medicines (with some exceptions, e.g. levomepromazine, ondansetron and octreotide
which should be diluted with normal saline) and unlikely to cause precipitation of medicines, but it is hypotonic and
may be associated with pain at the infusion site.3,7 However, in practice, pain is not that common because
of the slow rate of infusion.
Normal saline is also compatible with most medicines (with some exceptions, e.g. cyclizine which should be diluted with
sterile water) and may be less irritating at the insertion site because it is isotonic, however, the likelihood of precipitation
increases, particularly when more than one medicine is used.
Compatibility of medicines
When more than one medicine is used in an infusion solution there is a risk that they may not be compatible, either
chemically or physically. Increasing the number of medicines in the solution increases the risk of problems with the combinations.
Physical incompatibility usually results in changes in the solution that can be observed such as discolouration, clouding
or precipitation of crystals or particles. However, it is important to refer to compatibility tables because a solution
can remain clear even if the medicines are chemically incompatible.
Precipitation may occur as a result of a reaction between medicines in a syringe. The risk of precipitation can be minimised
by using sterile water as the diluent and by maximising the total volume of the solution in the syringe, i.e. making the
solution as dilute as possible.7
Once mixed, syringes should be observed for any signs of precipitation or discolouration. Provided that doses are within
normal ranges, Table 2 shows which injectable medicines are expected to be compatible in a 24-hour syringe driver solution.
Prescribing the medicines for the syringe driver
Convert the patient's previous 24-hour oral medicine requirements (including regular and "as needed" doses) to the equivalent
subcutaneous dose.
Usual starting doses for subcutaneous infusion for commonly used medicines are:
- Morphine - use half the total 24 hour oral dose
- Oxycodone - use half the total 24 hour oral dose
- Metoclopramide, cyclizine and hyoscine hydrobromide (the injectable hyoscine salt Buscopan) - same as the oral dose
- Haloperidol - antiemetic dose is 1 - 2 mg for 24 hours
- Midazolam - 5 - 40 mg over 24 hours
For patients who have not been on opioid medicine for analgesia, an example of an initial starting dose would be 10
mg morphine subcutaneously over 24 hours.3
Prescribe the doses of the subcutaneous medicines to cover a 24-hour period. Check the compatibilities of the medicines
in the syringe using the chart in The Palliative Care Handbook 2012 or Table 2 and decide on the volume to infuse, stating
the diluents. A maximum of 24 mL solution in a 30 mL syringe is appropriate for the Niki T34 syringe pump. Smaller syringes,
e.g. 10 mL and 20 mL, can also be used, but they should be filled to a maximum of 8 mL and 18 mL respectively. A luer-lock
syringe should always be used to avoid any risk of disconnection.
Larger volume syringes should be used for medicines that will require more ampoules to be combined to achieve the total
daily dose, e.g. metoclopramide, oxycodone and fentanyl, or medicines that are potentially irritant when given subcutaneously,
e.g. cyclizine, methadone and high doses of dexamethasone.
The first syringe of a new prescription will lose some of the solution when the line is primed, therefore the infusion
will not run for a full 24 hours. An initial subcutaneous injection may also be required as a loading dose to manage the
patient's symptoms for the initial two to four hours of syringe driver use until the medicines in the infusion reach effective
blood plasma levels. When an infusion is due to be changed, a delay of an hour or two should not cause problems if the
patient's symptoms are well controlled. This can be a concern for patients and families if the clinicians or nurses visit
is delayed.
Hospices and residential aged care facilities are likely to have standardised prescribing and administration charts
for syringe driver prescriptions. Similar documentation is recommended for patients who are receiving care at home.
 An example of a prescription chart for documenting medicines given via
syringe driver is available at: http://palcare.streamliners.co.nz click
on "forms".
An example of a prescription chart for documenting medicines given via
syringe driver is available at: http://palcare.streamliners.co.nz click
on "forms".
The individual medicines to go in the syringe can be prescribed on a standard prescription for a community pharmacy.
Indicate the prescription is for a syringe driver. State the dose and diluents, and remember a triplicate controlled drug
prescription for any opioids. Some community pharmacies provide a service for compounding medicine solutions in daily
subcutaneous syringes.
Administration instructions do not need to include the rate of infusion, just the infusion duration (usually 24 hours).
This is because the Niki T34 syringe driver simplifies administration by detecting the syringe size and volume of medicine,
and sets the rate to deliver the infusion over the required time period, e.g. 18 mL in a 20 mL syringe will deliver at
0.75 mL per hour for a 24-hour period.
Controlled drugs that are no longer required for a patient can be returned to the pharmacy or general practice for safe
disposal.
Starting the infusion
In most cases, a healthcare professional trained in the use of syringe drivers, e.g. a hospice nurse, district nurse
or residential aged care facility nurse, will assist with setting up the continuous subcutaneous infusion.
 A step by step guide for operating the Niki T34 is available from the
manufacturer, REM Systems. Instructions are also available online from many hospices.
A step by step guide for operating the Niki T34 is available from the
manufacturer, REM Systems. Instructions are also available online from many hospices.
Selection of the infusion site
Plastic cannulae are recommended, although metal butterfly needles can be used. The preferred sites for insertion of
the cannula for a continuous subcutaneous infusion are:
- The anterior chest wall
- The anterior abdominal wall
- The anterior aspect of the upper arms
- The anterior aspect of the thighs
These sites are preferred because they are accessible, both for initial insertion and for monitoring, and they are rarely
oedematous.3 The choice of site may be influenced by a number of factors including patient preference, their
level of mobility and the patient's condition, e.g. if they are cachexic the abdomen may be the most suitable site, the
upper arm should be avoided if the patient is bed-bound and requires regular turning and anterior sites may not be suitable
for patients who are agitated as they may dislodge the cannula - a posterior site over the scapula may be preferable.
Inappropriate sites include:3,5
- Lymphoedematous or ascitic areas - as absorption will be reduced and there is an increased risk of infection and leakage
- Areas of skin that are scarred, broken, inflamed, infected or hairy
- Skin folds or skin over bony prominences or near joints
- The anterior chest wall in patients who are very cachexic - there is a small risk of pneumothorax
- The upper abdomen in a patient with an enlarged liver - there is a small risk of puncturing the liver capsule
- Skin that has been irradiated within the last six weeks
- Any area that has a tumour
Minimising reactions at the site of insertion
A number of factors influence the longevity of the insertion site. These include the site selected, the type of cannulae
used and the medicine being given. If problems arise with an infusion site the patient may have localised discomfort,
or there may be reduced absorption of the medicine and a loss of symptom control. As a general guide, plastic cannulae
can stay in place for up to a week or more, whereas metal cannulae remain viable for approximately 72 hours.3,6 Provided
there is no evidence of a site reaction, it is reasonable to only change a site when it becomes necessary, e.g. due to
pain, swelling or inflammation.3,4
Techniques that may help to prolong the usefulness of a site and to minimise reactions include:
- Make the solution as dilute as possible - use a larger syringe
- When possible, select a solution that is close to physiological tonicity - sterile water is hypotonic, normal saline
is isotonic, and solutions with high concentrations of some medicines become hypertonic
- Use plastic cannulae as they cause less site irritation than metal cannulae
- In a patient who has been prone to site problems, consider rotation of the site of infusion before any localised reactions
develop
- Avoid oedematous areas when selecting the site for infusion
- Use 0.5 - 1 mg of dexamethasone in the syringe driver solution to reduce site reactions, particularly if the medicines
used are known to be irritant, e.g. methadone6
- Consider the use of heparinoid (Hirudoid) cream (not subsidised) on inflamed sites if there is no infection present6
Monitoring the infusion
Patients being cared for at home should ideally have a daily visit from a health professional for review of symptom
control and monitoring of the infusion. This should occur at least every four hours when patients are in a hospice or
residential aged care facility.
A check should be made of the:
- Cannula site - for redness, swelling, leakage or cannula blockage or displacement
- Tubing - for kinks or knots in the tubing
- Syringe - for precipitation or crystallisation, discolouration of solution
- Syringe driver - to ensure that the syringe remains in the correct position, that the infusion is running at the correct
rate and the syringe driver battery has enough power to last until the next check
Managing breakthrough symptoms
First check that the medicines are being delivered effectively via the syringe driver.
Breakthrough pain can be treated with additional subcutaneous doses of the opioid being used (usually morphine). If
possible, doses should be given through a side port in the syringe driver cannula line to minimise patient distress. This
can be given as often as required to relieve breakthrough pain. Doses can be prescribed in a flexible manner to achieve
good symptom control, e.g. 2.5 mg morphine as required every 15 minutes up to a total of three doses over 60 minutes.
Extra doses of antiemetics and other medicines in the syringe can also be given subcutaneously at the usual dose. If
supplementary doses are required regularly for breakthrough symptoms, include these doses when calculating the amount
of medicine needed for the subsequent 24 hour period. If the patient's symptoms remain uncontrolled despite an increase
in dose, consider an alternative medicine (e.g. because nausea may have many underlying causes it may be relieved by different
medicines) and consider a discussion with a palliative care physician. Also consider other methods to relieve a patient's
distress - sometimes taking the time to sit and listen can be as effective as administering a medicine.
Further resources
 General Practitioners and other carers can access 24-hour telephone
help from their nearest hospice:
www.hospice.org.nz/find-your-local-hospice-service
General Practitioners and other carers can access 24-hour telephone
help from their nearest hospice:
www.hospice.org.nz/find-your-local-hospice-service
 The Palliative Care Handbook, Guidelines for clinical management and
symptom control. 6th Edition, 2012 is available as a printed copy (yellow book) free-of-charge from any hospice or download
an electronic version from: www.hospice.org.nz
The Palliative Care Handbook, Guidelines for clinical management and
symptom control. 6th Edition, 2012 is available as a printed copy (yellow book) free-of-charge from any hospice or download
an electronic version from: www.hospice.org.nz