What constitutes intermediate hyperglycaemia?
Diabetes is diagnosed with an HbA1c level of 50 mmol/mol or greater (and, if measured, a fasting blood glucose
≥7.0mmol/L).1,2 This is the glycaemic level at which the incidence of moderate diabetic retinopathy begins
to rise exponentially.2 In an asymptomatic person, a result greater than 50 mmol/mol should be repeated to
confirm the diagnosis.
Intermediate hyperglycaemia is a biochemical state in which a person has glucose levels above the normal range, but
does not yet meet the criteria for a diagnosis of diabetes. In New Zealand intermediate hyperglycaemia is defined as an
HbA1c level of 41 - 49 mmol/mol (and, if measured, a fasting blood glucose of 6.1 - 6.9 mmol/L).1,2
Unlike diabetes, repeat testing is not required to confirm intermediate hyperglycaemia. A single HbA1c result
between 41 - 49 mmol/mol in a person who is not acutely ill or who does not have a condition* that may affect HbA1c levels
is sufficient. HbA1c should then be monitored every six to 12 months, unless there are intervening symptoms.2
* For example high red blood cell turnover, iron or B12 supplementation or recent blood transfusion can falsely lower
HbA1c levels and severe anaemia, B12 or folate deficiency, chronic alcoholism, chronic renal failure or certain
haemoglobinopathies can falsely raise HbA1c levels.
HbA1c is most frequently requested in the context of a cardiovascular disease (CVD) risk assessment. If a
person is identified as having intermediate hyperglycaemia, and they are in the targeted age range, they should have a
full CVD risk assessment, including investigation of lipid levels, if this was not done at the same time as the HbA1c test.
In New Zealand, normoglycaemia is defined as an HbA1c level below 40 mmol/mol (and, if measured, a fasting
blood glucose ≤6.0mmol/L).1,2
 For further information on the use of HbA1c to diagnose diabetes,
see: "The new role of HbA1c in diagnosing type 2 diabetes",
BPJ 42 (Feb, 2012) and "Understanding the new HbA1c units for the diagnosis of Type 2 diabetes", Braatvedt
G et al, NZMJ 2012;125(1362).
For further information on the use of HbA1c to diagnose diabetes,
see: "The new role of HbA1c in diagnosing type 2 diabetes",
BPJ 42 (Feb, 2012) and "Understanding the new HbA1c units for the diagnosis of Type 2 diabetes", Braatvedt
G et al, NZMJ 2012;125(1362).
Intermediate hyperglycaemia is an independent risk-factor for diabetes
Approximately 5 - 10% of people with intermediate hyperglycaemia progress to diabetes every year, but 5 - 10% will revert
back to normogylcaemia.5,6 However, in the long-term, up to 70% of people with intermediate hyperglycaemia
will eventually develop type 2 diabetes.7 This represents a relative risk for type 2 diabetes six times greater
for people with intermediate hyperglycaemia than those with normogylcaemia.5
Intermediate hyperglycaemia should be viewed as a continuous scale rather than a discrete variable, i.e. those at the
higher end of the 41 - 49 mmol/mol range have a much greater risk of developing diabetes than those at the lower end.
The risk of having a high glycaemic level remains, even for people who have undergone proven prevention such as bariatric
surgery.8
In addition, other factors play an important part in assessing an individual's risk of progressing to diabetes, including:
- Age
- Weight and BMI
- Physical activity
- Diet
- Ethnicity - Māori, Pacific and South-Asian people have an increased risk of diabetes
- Smoking status - three-fold increase in risk of progression to diabetes
- Family history of diabetes
- Increased blood pressure
- Increased lipid levels
- Certain conditions, such as polycystic ovary syndrome (see below)
There are a number of tools for assessing who is at risk of developing diabetes, and helping to determine the pre-test
probability of a diagnosis in people who have not had a recent HbA1c test. The most practical tools incorporate
information readily available to primary care and have a high specificity for future diabetes progression.9 These
prediction tools have not been validated in high risk ethnicities in New Zealand, therefore a single tool cannot be recommended
above any other for this population. However, viewing the patient's risk in light of the above risk factors is likely
to be beneficial in assessing the likelihood of progression to diabetes.
Many vascular complications begin before HbA1c reaches "diabetic" levels
While diabetes is defined by the threshold where the prevalence of moderate diabetic retinopathy begins to increase
exponentially, many of the underlying pathophysiological processes associated with diabetes begin during the intermediate
hyperglycaemic stage. This includes nephropathy, chronic kidney disease, neuropathy, retinopathy, cardiovascular disease
and overall mortality.10 Similarly, increased vascular risk remains even in people with diabetes who have achieved
target glycaemic levels below the diagnostic threshold for diabetes.11
Early management is particularly important among high-risk groups as complications develop earlier, often before a diagnosis
of diabetes is made, although this could be a result of either lower testing rates (meaning longer unmanaged time with
hyperglycaemia) or a greater actual risk. For example, in a New Zealand study of 4269 Māori, of the participants
identified as having clinical diabetes, 29.6% already had established microalbuminuria, and 7.7% albuminuria, at diagnosis.12
Management of intermediate hyperglycaemia reduces progression to diabetes
The identification and management of people with intermediate hyperglycaemia should be viewed as an opportunity to halt
progression to diabetes and to reduce the risk of diabetes related complications. Intervention with lifestyle changes,
and initiation of metformin where appropriate, can reduce the number of people progressing to clinical diabetes by 30
- 60%.6,13 Treatment is considered to be both safe and cost-effective.13-15
The Diabetes Prevention Program, a large United States study of early prevention, found that 5% of people in the lifestyle
intervention group and 7.8% of people in the metformin group (no lifestyle changes) developed clinical diabetes each year,
compared to 11% of those in the placebo group (Figure 1).6 Lifestyle intervention was more advantageous than
metformin in people aged over 60 years and those with a lower body-mass index.6 People who achieved even one
normoglycaemic result during the study period had a significantly lower long-term risk of progressing to diabetes.16
In the Diabetes Prevention Program, women with a history of gestational diabetes and current intermediate hyperglycaemia
showed significant benefit from both lifestyle intervention and metformin. Each intervention reduced the relative risk
of progression to diabetes by approximately 50% compared with the placebo group. In contrast, this reduction was 49% for
lifestyle and 14% for metformin in women without a history of gestational diabetes.17
Overall, it is estimated that the number of people with intermediate hyperglycaemia who need to be treated for three
years in order to prevent one case of diabetes is 6.9 for lifestyle intervention and 13.9 for metformin.6
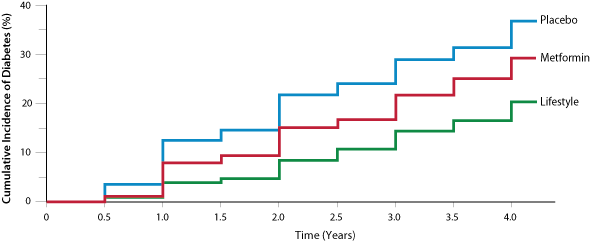 Figure 1
Figure 1: The cumulative incidence of diabetes based on intervention
6
Treatment goal: agree on a target HbA1c level
There is no defined treatment target in people with intermediate hyperglycaemia, therefore a target should be individually
agreed upon between the patient and the doctor. Ideally the goal of treatment should be regression to normoglycaemia,
in which case an HbA1c target of < 40 mmol/mol should be aimed for, as this is associated with significant
reductions in long-term diabetes risk.15 However, for many people delaying and slowing the progression to diabetes
is a more realistic target and is still likely to be beneficial.
Repeat testing of HbA1c is recommended every 6 - 12 months, unless there are intervening symptoms.20
N.B It is critical that HbA1c level targets are not the only goal set, weight loss and exercise goals should
be part of the treatment process.
Lifestyle intervention for everyone
Most people with raised glycaemic levels will benefit from advice on an intensive programme of lifestyle changes.4 The
two most important modifiable risk factors for diabetes development are obesity and physical inactivity.7 Target
goals should be set for weight, weekly physical activity level and diet, e.g. fat intake, fibre intake and total kilojoules.
Key advice includes encouraging people to:4
- Undertake a minimum of 150 minutes of "moderate intensity" physical activity per week
- Gradually lose weight to reach and maintain a BMI within the healthy range
- Increase consumption of whole grains, vegetables and other foods that are high in dietary fibre
- Reduce the total amount of fat in their diet
- Eat less saturated fat
In the Finnish Diabetes Prevention study, achievement of lifestyle intervention goals resulted in a reduction of 58%
in the risk of progression to diabetes.18 Achieving each individual goal (e.g. exercise, reducing fat in the
diet) was independently associated with reduced risk of progression to diabetes. For example, a 5% reduction in weight
significantly reduced the overall risk of developing diabetes (odds ratio of 0.3 compared to no weight loss).18 In
the Diabetes Prevention Program study population, it was found that even a small weight loss, e.g. 1 kg, resulted in a
significant reduction in the risk of progression to diabetes.6
Some practices may consider addressing lifestyle interventions with patients in a group setting. Key components of a
successful programme include:4
- Participants meet at least eight times over 9 - 18 months
- Participants have at least 16 hours of educational time, either in the group or on a one-to-one basis
- Follow-up sessions should be offered regularly, e.g. every three months, for at least two years after the programme
- Behavioural change techniques should be used in conjunction with diet and exercise advice, e.g. setting short and
long-term goals, identifying triggers
Referral to a dietitian for further advice and guidance is likely to be beneficial, although waiting times for publically
funded services and the cost of private services may be an issue for some people.
 For further information on lifestyle interventions see: "Addressing
weight issues in young people and families in New Zealand". BPJ 45 (Aug, 2012) and "Promoting
healthy lifestyles for Pacific peoples" BPJ 32 (Nov, 2010).
For further information on lifestyle interventions see: "Addressing
weight issues in young people and families in New Zealand". BPJ 45 (Aug, 2012) and "Promoting
healthy lifestyles for Pacific peoples" BPJ 32 (Nov, 2010).
Add metformin if lifestyle intervention is insufficient
After approximately six months of lifestyle intervention, people who do not show glycaemic improvement, gain weight
or are unable to adhere to recommendations may be offered metformin as an adjunct to lifestyle intervention.
In addition, most women with polycystic ovary syndrome and raised glycaemic levels are likely to benefit from a trial
with metformin (see "The role of metformin in women with polycystic ovary syndrome").19 Women with a history
of gestational diabetes and current intermediate hyperglycaemia are also likely to particularly benefit from metformin.17
Because the benefits of metformin are not maintained after cessation, it is important that diet and lifestyle management
is continued during metformin treatment in order to address and improve the underlying obesogenic behaviours that contribute
to intermediate hyperglycaemia and diabetes. Long-term primary prevention can then be maintained even after medicine cessation.
The role of metformin in women with polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is a group of disorders that result in inappropriate gonadotropin secretion, usually
from the ovaries but technically from anywhere along the hypothalamic-pituitary-ovarian (HPO) axis. It is common, affecting
between 5 - 10% of women of reproductive age.19
The syndrome is characterised by a varying combination of menstrual dysfunction (typically oligo-ovulation or anovulation
manifested by oligoamenorrhea), hyperandrogenism and polycystic ovaries on ultrasound. Insulin resistance is common in
women with PCOS, along with an increased risk of developing type 2 diabetes and cardiovascular disease. Annual HbA1c testing
is recommended, but there is very little evidence for measurement of plasma insulin in women with PCOS.
The role of metformin in women with PCOS is controversial. Metformin is potentially useful for maintaining satisfactory
glycaemic levels and in increasing fertility, although evidence of effect has been difficult to find until recently.
The PCOSMIC trial, a New Zealand trial investigating the use of metformin and clomiphene in women with PCOS, found that
metformin increased the pregnancy rate, and that clomiphene plus metformin was more effective than either metformin or
clomiphene alone.21 A long-term United States study found that metformin improved the metabolic profile of
women with PCOS over a 36-month treatment course, particularly improving circulating HDL-cholesterol, diastolic blood
pressure and BMI.19
At present, there is no recommendation to treat all women with PCOS with metformin, nor is it an approved indication
for metformin. However, women with PCOS and an HbA1c level > 40 mmol/mol are likely to benefit from use of
metformin, in addition to lifestyle interventions.
How to prescribe metformin for intermediate hyperglycaemia
 Intermediate hyperglycaemia is not an approved use for metformin, but
it can be prescribed fully subsidised for this indication and there is strong clinical evidence to justify its use.
Intermediate hyperglycaemia is not an approved use for metformin, but
it can be prescribed fully subsidised for this indication and there is strong clinical evidence to justify its use.
Metformin can be commenced at a low dose, e.g. 500 mg once daily, and increased gradually, e.g. over several weeks,
as tolerated to a maximum of 2 g daily if required.4
Lactic acidosis has been reported in people taking metformin who have reduced renal function. Although the risk may
be overstated, at present it is recommended that the dose of metformin should be reduced if renal function deteriorates,
and people who decline to an eGFR < 30 mL/min/1.73m2 or are at risk of rapid decline in renal function should
cease treatment.1 A Cochrane review of 96,295 patients found no incidences of lactic acidosis in people taking
metformin, even in those who had a contraindication to metformin, such as renal insufficiency.22
Consider a maximum daily dose of 1 g metformin in patients with eGFR 30-60 mL/min/1.73m2. However, in patients
who are obese, eGFR may be an underestimate of their true creatinine clearance, and this group is likely to benefit most
from metformin. Therefore metformin doses may not have to be reduced in obese patients with eGFR in the 45-60 mL/min/1.73m2 range.
If required, consider using the Cockcroft-Gault equation to calculate a more accurate value for creatinine clearance in
these patients. As metformin is primarily cleared by the kidneys, all patients taking metformin should have their renal
function monitored annually and more frequently if clinically indicated.
 For further information on eGFR and kidney disease, see "Making
a difference in chronic kidney disease". BPJ 22 (Jul, 2009) and "Acute-on-chronic
kidney disease: prevention, diagnosis, management and referral in primary care", BPJ 46 (Sept, 2012).
For further information on eGFR and kidney disease, see "Making
a difference in chronic kidney disease". BPJ 22 (Jul, 2009) and "Acute-on-chronic
kidney disease: prevention, diagnosis, management and referral in primary care", BPJ 46 (Sept, 2012).
Compliance and adverse effects of metformin need to be monitored
Metformin is generally well tolerated. The most common reported adverse effect is gastrointestinal disturbance. Other
adverse effects include anorexia, nausea, taste disturbance and decreased vitamin-B12 absorption.
Regularly follow-up to ensure that metformin is being taken as prescribed and that adverse effects are not affecting
adherence.
Metformin may need to be continued long-term
Metformin should be given initially for six to 12 months.4 For many people metformin will need to be continued
long-term. If no effect is seen with metformin, consider stopping the medicine, but continue with lifestyle interventions.4 If,
despite metformin treatment, the patient progresses to diabetes, other medicines, e.g. sulphonylureas or insulin, may
need to be added if glycaemic control cannot be achieved with metformin alone.