The framework for lipid modification at younger ages is extrapolated for those over 75. The largest trial conducted
in this group is the PROSPER trial with over 5000 participants, between 70 and 82 years old, and followed up for an average
of 3.2 years.3 It is used as the basis of most recommendations for lipid lowering treatments in older populations.
There is modest but clear prevention of cardiovascular mortality and morbidity using the primary composite endpoint
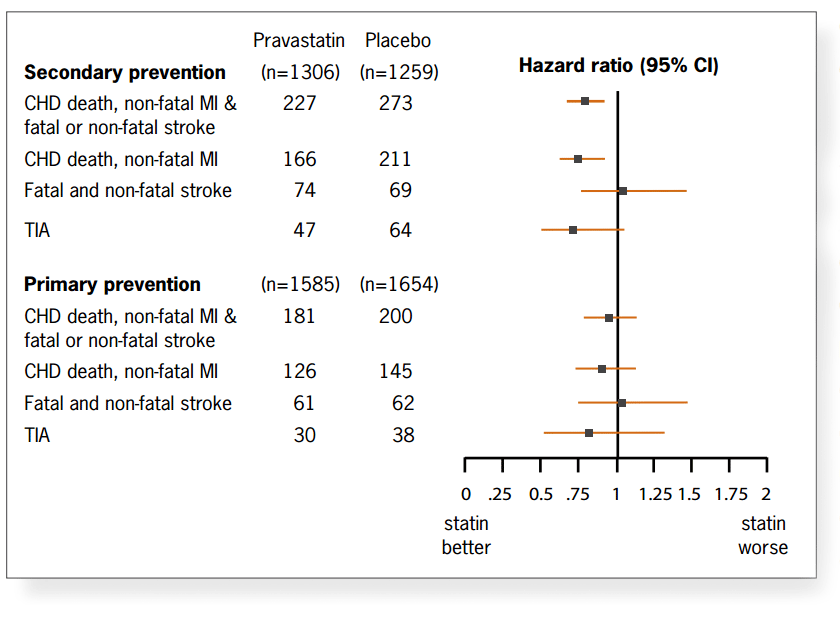
of CHD death or non-fatal MI (absolute risk reduction 2.1%, NNT 48). Figure 2 shows the primary and secondary outcomes
underpinning the claims for the benefits of pravastatin over placebo for preventing cardiovascular morbidity and mortality.
For women, the results showed no benefit over placebo for any outcome. There is also no demonstrable benefit in primary
prevention yet the conclusions are that the strategy for cardiovascular risk management in middle aged people should also
be applied to elderly individuals.
Figure 2: Major cardiovascular outcomes, according to primary or secondary prevention
status of participants
The primary endpoint of the study is reproduced for comparative purposes.
If the detail of the paper is examined, the other morbidity and mortality data are illuminating. Despite a change in
cardiovascular composite outcomes, there is no change in all cause mortality (Figure 3). Looking at mortality and morbidity
from other causes, rates of cancer diagnosis and death were increased in the treatment group compared with placebo. This
did not quite reach significance for death but did for new diagnosis of cancer (NNH 59).
Figure 3: Outcome
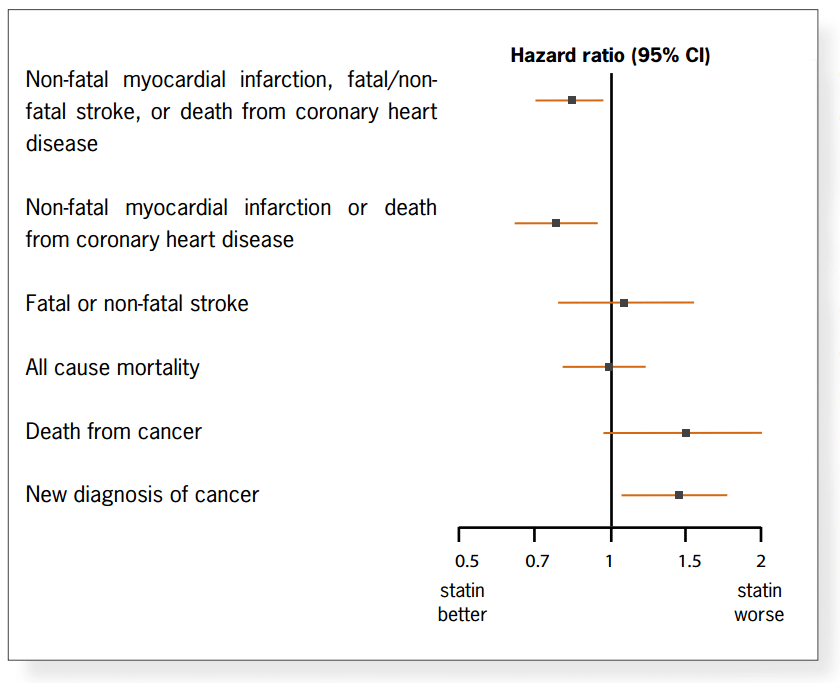
The efficacy of a treatment might be justified in terms of postponement of morbidity even when there is no change in
mortality, in the PROSPER paper, when the scrutiny of treatment outcomes is extended beyond a single disease model, this
argument does not hold. The increase in new cancer diagnoses counters any arguments of compression of morbidity. The more
likely hypothesis for this effect, which is not seen in trials of younger patients, is substitution of cause of death.
This is a phenomenon which is unprecedented. The prevention of untimely death is a valid pursuit of medicine up to a
point. Thus when we vaccinate children in infancy we are selecting out a cause of death for them, but in this case justifiably,
because deaths from infectious disease tend to occur prematurely. It is only when we start selecting out causes of death,
rather than extending life, that this endeavour becomes questionable.
Many patients fear the manner of their dying more than death itself and many regard coronary heart disease as a 'good
way to go' in old age.5 In prescribing medicines to prevent particular diseases, we may select for another
cause of death unknowingly and certainly without the patient's informed consent. This is fundamentally unethical, undermining
the principle of autonomous choice and the concept of 'primum non nocere'. An older patient who has elected to 'reduce
the risk of heart attack' may make a different decision when told 'you will not extend your years of life and you will
increase your risk of being diagnosed with and dying of cancer'
Clinical decisions about prescribing for disease prevention carries additional responsibilities.6
Preventive treatments do not relieve suffering directly, but are designed to reduce some future risk of suffering
and are usually initiated by the suggestion of the physician rather then a patient request. Compared with initiation
of treatment to relieve suffering, a degree of persuasion is involved in starting preventive treatments.7 As
clinicians we must therefore be reasonably certain they will fulfill their promise. There are harms other than the side
effects of the actual treatments, not the least of which is the shadow cast over a currently healthy life by the threat
of disease. One might argue that this particular harm is magnified as mortality looms closer. These considerations may
explain the evident discomfort of general practitioners and their apparent reluctance to follow guidelines for cholesterol
measurement and lipid lowering agents in those aged 75 years or over, compared with those under 75.8 While
every treatment decision is an individual one, guidance for populations is based on population data. The PROSPER study
has been acknowledged as the best available evidence for the effects of statins for prevention of cardio- and cerebrovascular
disease in old age. We cannot ignore it.
The best interests of the older person, who has paid a lifetime of taxes, might be to invest that money in health care
that will genuinely relieve their suffering. Cataract and joint replacement surgery, and personal care of those with dementia,
provide obvious examples. A different model is required for assessing medicines for prevention in old age. One that includes
duration of life extension, duration of treatment and takes account of mortality and morbidity, due to all causes as well
as the harms attributable to treatment. Some preventive interventions that have benefits across a range of conditions
will likely be of similar benefit in older populations as in younger populations using this model (flu vaccination, exercise,
smoking cessation). Some may be of more benefit in older populations, some like statins, will be of less benefit.
Patients and physicians are entitled to all the information they require to make decisions with such profound consequences.
The current international single disease models of research, analysis and guidelines make this unlikely. Evidence for
older populations cannot be squeezed uncomfortably into models designed to best assess the benefits and harms of treatment
in younger populations. Our current models of quality usually relate to 'doing something'. A better model would acknowledge
confidently when not to use medicines - when best practice is 'not doing'. If we continue using the current framework
the benefits will accrue only for pharmaceutical companies with increasing profits from an ageing population consumed
by epidemics rather than enjoying their long life.