The burden of COPD
Chronic Obstructive Pulmonary Disease (COPD) is estimated to affect 15% of all New Zealanders aged over 45 years.1 It
is the fourth leading cause of death in New Zealand behind cancer, heart disease and stroke.1 COPD is permanent,
disabling and frequently progressive. Over 85% of cases of COPD are caused by inhalation of tobacco smoke.1
COPD in Māori and Pacific peoples
Amongst New Zealanders aged 50 – 64 years, Māori are approximately five times more likely to die from COPD-related
causes than non-Māori and are affected by COPD up to 20 years earlier.2,3 COPD is ranked as the third
highest health priority for Pacific peoples in the Auckland DHB region.4
There is also evidence that COPD may be under-diagnosed in New Zealand, especially among Māori. In a study of
3500 randomly selected people aged over 25 years in the greater Wellington area, 736 people were referred for pulmonary
testing. Overall, 16% of those tested had COPD, and 23% of Māori in the group had COPD.5
Hospital discharge rates (Figure 1) show that Māori and Pacific peoples are three to four times more likely to
be admitted to hospital for COPD than people in other ethnic groups in New Zealand.6
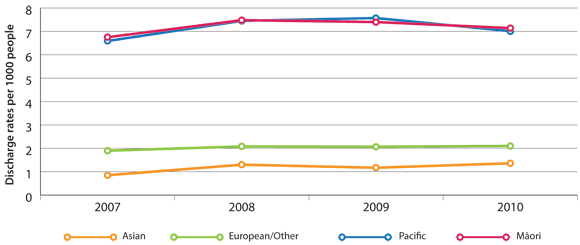
Figure 1: Age-standardised hospital discharge rates in New Zealand with a primary diagnosis of COPD,
per 1000 enrolled patients by ethnicity*
* Data source: Ministry of Health, National Minimum Data Set (NMDS). Hospital Discharges Population source:
DHB Estimated Resident Population 1996-2006 (Prioritised)
Promoting awareness may encourage Māori and Pacific peoples with COPD to contact their general practice earlier.
Some patients may delay visiting their doctor due to the slowly progressive nature of the disease, while others may
be dismissive of their symptoms, e.g. just a "smoker's cough" or "just being older and unfit". Financial barriers to
accessing services are also likely to contribute.7
Every Māori or Pacific person who is a current, or ex-smoker, or has household members that smoke should be made
aware of the:
- Support that is available to help them stop smoking
- Symptoms of COPD and the need to visit a health professional if a family member displays symptoms
- High impact COPD has on Māori and Pacific communities
Once a person has symptoms of COPD, lung damage has already occurred. This damage cannot be reversed, but can be substantially
slowed through smoking cessation and prevention of exacerbations.
"Support not blame" is an important approach for health professionals engaging with people who have COPD. A feeling
of judgement or blame, because of the association between smoking and COPD, may cause people to present to their general
practice later than they otherwise would have. General practice staff need to be seen as welcoming and supportive.
Testing for COPD
Spirometry
Spirometry is the recommended method for diagnosing COPD.8 Ideally this should be performed with a device
that allows electronic analysis of results (see "Spirometry devices"). International guidelines recommend that spirometry
should be offered to any person aged over 40 years with any of the following characteristics:2
- Chronic cough (may be sporadic and unproductive)
- Chronic sputum production (phlegm)
- Dyspnoea that is persistent or progressive and worse with exercise
- History of exposure to tobacco, occupational smoke, dust or chemicals
- Family history of COPD
Due to the earlier age of onset and increased burden of COPD in Māori and Pacific peoples, testing for COPD in
selected people who are at increased risk should begin at a younger age, e.g. Māori or Pacific peoples who are
heavy smokers and have a family history of COPD may benefit from being offered testing from age 30 years.
It is important that normal spirometry results are not interpreted by the patient as a disincentive to stop smoking.
Conversely, early detection and the "shock" of a diagnosis of COPD often helps to motivate people to stop smoking.9
 Asthma Societies throughout New Zealand provide spirometry and support
services for people with COPD. A list of local branches of Asthma Societies is available from:
Asthma Societies throughout New Zealand provide spirometry and support
services for people with COPD. A list of local branches of Asthma Societies is available from:
www.asthmafoundation.org.nz/asthma_societies.php
If spirometry is unavailable, questionnaires, such as the clinical COPD questionnaire (CCQ), may be used as an indication
of the likelihood of COPD.
 The clinical COPD questionnaire is available from: http://ccq.nl/
The clinical COPD questionnaire is available from: http://ccq.nl/
Spirometry testing should be performed when the patient is clinically stable and without infection. Patients should
be advised not to use a short-acting bronchodilator in the six hours prior to testing, or a long-acting bronchodilator
in the 12 hours prior to testing.
Values should be measured:
- Before and 10 – 15 minutes after administering a short-acting beta-2 agonist, e.g. salbutamol 400 micrograms (four
puffs) via a spacer
- or
- Before and 30 – 45 minutes after administering a short-acting anticholinergic, e.g. ipratropium 160 micrograms (eight
puffs) via a spacer
A diagnosis of COPD is defined as a post-bronchodilator forced expiratory volume (in one second) to forced vital capacity
ratio (FEV1/FVC) of < 0.7.10
The peak expiratory flow rate (PEFR) should not be used in the diagnosis or management of COPD as it is a measure
of airflow in large airways.
Spirometry devices
Equipment of a high standard is required to take accurate spirometry measurements. Software should display a real-time
flow volume graph adjusted for body temperature. Results should be able to be printed and the equipment easily dismantled
for cleaning and disinfection. Flow-based spirometers need to be regularly checked with a calibrated syringe. Training
in the use of a spirometry device is recommended.
 The Asthma Foundation provides information on spirometry courses for
health professionals. A list of recommended portable spirometers is available from:
The Asthma Foundation provides information on spirometry courses for
health professionals. A list of recommended portable spirometers is available from:
www.asthmafoundation.org.nz/spirometry.php
Assessing the severity of COPD
Assessment of COPD severity should take into account the following:2
- Level of breathlessness (Table 1)
- Spirometry results (Table 2)
- Exacerbation risk – calculated from the number of exacerbations experienced in the previous 12 months: less than
two exacerbations is low risk, two or more is high risk
- Presence of co-morbidities – influences risk of hospitalisation and overall mortality risk
| Table 1: Modified Medical Research Council questionnaire for assessing breathlessness.2 |
| Grade |
Description of breathlessness |
| 0 |
Only gets breathless after strenuous exercise |
| 1 |
Gets short of breath when hurrying on the level or walking up a slight hill |
| 2 |
Walks slower than people of the same age due to breathlessness, or has to stop for breath when
walking at own pace on the level |
| 3 |
Stops for breath after walking for 100 m or after a few minutes on the level |
| 4 |
Too breathless to leave the house, or breathless when dressing |
| A symptom grade of 0 or 1 indicates few symptoms, a grade ≥ 2 indicates a high level of symptoms |
| Table 2: Classification of airflow limitation severity in patients with FEV1/FVC < 0.7.2 |
| Classification |
Post-bronchodilator FEV1* |
| Mild |
≥ 80% Predicted |
| Moderate |
≥ 50% to < 80% Predicted |
| Severe |
≥ 30% to < 50% Predicted |
| Very severe |
< 30% Predicted |
| *Most spirometers provide predicted values from healthy population studies that account for height,
age and gender |
Rates of smoking in Māori and Pacific peoples are unacceptable
In 2009, 21% of New Zealanders aged 15 – 64 years were current smokers.11 Smoking rates were higher for
Māori (males 40.2%, females 49.3%) and Pacific peoples (males 32.3%, females 28.5%) in the same age group.11 This
is the primary reason Māori and Pacific peoples are disproportionately affected by COPD. Encouragingly, survey
results show that smoking rates among younger Māori and Pacific peoples may be decreasing. The 2011 ASH smoking
survey found that Māori secondary school students had the greatest decline in daily smoking rates, with a change
from 14.1% in 2010 to 10.3% in 2011. Rates among Pacific students reduced from 7.0% to 5.9%.12
Aukati Kaipaipa is a face-to-face smoking cessation service that is accessible in most communities. The programme
provides Māori access to NRT, motivational counselling and other activities. A list of providers is available on
the Aukati KaiPaipa website: www.aukatikaipaipa.co.nz/contact-us
 For further information see: "Smoking
cessation for Māori", BPJ 22 (Jul, 2009)
For further information see: "Smoking
cessation for Māori", BPJ 22 (Jul, 2009)
Training to provide smoking cessation support for Pacific peoples is provided by the Heart Foundation. This consists
of one day of theory training and one day of follow-up support. For further information see: www.heartfoundation.org.nz/programmes-resources
A new approach to smoking cessation using a "buddy" system
People with COPD who continue to smoke often feel judged. This perception may prevent people attending consultations,
accessing treatment or even leaving their home. A recent small trial in a deprived area of the United Kingdom has shown
some success in overcoming this.13
People with COPD who have successfully stopped smoking are assigned as "buddies" to other motivated people with COPD
who are current smokers. The "buddies" are given brief training in smoking cessation and then provide support and encouragement
for their "buddy" during the cessation attempt. Results from the programme in 2011 showed that of 30 people who had
used this support service, the four-week abstinence rate was over 80% and abstinence at 12 months was 50%. This compares
to abstinence rates of 44% and 23% respectively for other smokers using varenicline and psychosocial support to aid
their cessation attempt.14
This experience from the United Kingdom is an example of whānaungatanga (togetherness and support) being applied
to smoking cessation and may be directly transferable to Māori and Pacific communities in New Zealand.
Management of COPD
Once a diagnosis of COPD has been made, there is strong evidence that smoking cessation reduces the rate of lung function
decline.10 People with COPD who smoke, typically smoke more cigarettes per day than other smokers and have a comparatively
higher physical dependence to nicotine.15 Motivating a person with COPD to stop smoking (i.e. using "ABC" – ask, brief
advice, cessation support) should be the primary management focus, followed by pharmacological treatment, pulmonary
rehabilitation and management of exacerbations.
 For further information on smoking cessation see: "Update on smoking
cessation", BPJ 33 (Dec, 2010).
For further information on smoking cessation see: "Update on smoking
cessation", BPJ 33 (Dec, 2010).
Pharmacological treatment of COPD
Medicines for COPD are used to improve patient comfort and exercise tolerance while reducing the frequency of exacerbations.
No medicine currently available has been conclusively shown to modify the long-term decline in lung function associated
with COPD.2 Medicine choice should be based upon severity of symptoms and patient-specific response (Table 3). It is
important that patients and their whānau monitor symptoms in order to discuss management with their healthcare
team. If inhaled medicines are used, training in inhaler technique is essential and inhaler technique should be regularly
assessed.
 The Asthma Foundation website has printable booklets which provide
instructions on correct inhaler use, storage and cleaning. Available from: www.asthmafoundation.org.nz/resources.php
The Asthma Foundation website has printable booklets which provide
instructions on correct inhaler use, storage and cleaning. Available from: www.asthmafoundation.org.nz/resources.php
| Table 3: Recommended initial pharmacological treatment of COPD.2 |
| Severity |
First-line |
Second-line |
| Few symptoms and low risk of exacerbations |
Short-acting anticholinergic or SABA |
Combined short-acting bronchodilators or
Long-acting bronchodilator
|
| Many symptoms and low risk of exacerbations |
Long-acting anticholinergic or LABA |
Long-acting anticholinergic + LABA |
| Few symptoms and high risk of exacerbations |
ICS + LABA or
Long-acting anticholinergic |
Long-acting anticholinergic + LABA |
| Many symptoms and high risk of exacerbations |
ICS + LABA or
Long-acting anticholinergic |
ICS + LABA + long-acting anticholinergic |
Choosing a treatment regimen
The following points are generally applicable when selecting medicines for the management of stable COPD:2
- Inhaled bronchodilators are preferable to oral bronchodilators
- When symptoms are mild, short-acting bronchodilators are preferable to long-acting formulations
- When symptoms are more severe, long-acting bronchodilators are superior due to increased duration of action and
a reduction in the risk of exacerbations. There is no strong evidence to recommend one long-acting formulation over
another and treatment choice should be based on patient perception of symptom relief
- When COPD is severe and the risk of exacerbations is high, the addition of inhaled corticosteroids is indicated
- Suggested starting doses of medicines are listed in Table 4
| Table 4: Suggested starting doses of inhaled medicines for COPD10,16 |
| Medicine |
Dose per puff |
Number of puffs |
Frequency |
Delivery device |
| Beta-2 agonist |
| Salbutamol |
100 micrograms |
2 |
Four times daily as required |
MDI |
| Terbutaline |
250 micrograms |
2 |
Four times daily as required |
DPI |
| Salmeterol |
25 micrograms |
2 |
Twice daily |
MDI |
| 50 micrograms |
1 |
Twice daily |
DPI |
| Anticholinergic |
| Ipratropium |
20 micrograms |
2 |
Four times daily |
MDI |
| Titropium |
18 micrograms |
1 |
Once daily |
DPI |
| Combination inhalers |
| Salbutamol and ipratropium |
100/20 micrograms |
2 |
Four times daily |
MDI |
| Budesonide and eformoterol |
200/6 micrograms |
2 |
Twice daily |
DPI
(dose different for MDI) |
Fluticasone and salmeterol
|
125/25 micrograms |
2 |
Twice daily |
MDI
(dose different for DPI) |
| Corticosteroid |
| Budesonide |
400 micrograms |
1 |
Twice daily |
DPI |
| Fluticasone |
250 micrograms |
2 |
Twice daily |
MDI or DPI |
MDI = metered dose inhaler, DPI = dry powder inhaler
Short-acting beta-2 agonists (SABA), e.g. salbutamol, terbutaline, are usually prescribed as "rescue" medicine
(as required) for the relief of breathlessness.
Short-acting anticholinergics, e.g. ipratropium, have been shown to improve quality of life and decrease
the need for oral corticosteroid treatment, while decreasing the risk of adverse effects compared to SABA.10
Short-acting combinations, e.g. salbutamol and ipratropium, have been shown to improve spirometry
results and reduce the need for oral corticosteroids, compared to ipratropium alone.10
Long-acting beta-2 agonists (LABA), e.g. salmeterol, reduce exacerbations and improve symptoms, and
are more effective at maintaining symptom relief than SABAs. They are effective for at least 12 hours and can be administered
twice daily.
Long-acting anticholinergics, e.g. tiotropium, reduce exacerbations and have an effect over 24 hours
and are therefore administered once daily. There is an increased risk of dry mouth and urinary retention. Tiotropium
is fully subsidised under Special Authority.
Inhaled corticosteroids (ICS), e.g. budesonide, fluticasone, decrease the exacerbation rate compared
to placebo, and increase quality of life,2 but do not appear to prevent lung function deterioration and may
increase the risk of pneumonia.12 Systemic absorption does occur and long-term use must be balanced against the risk
of adverse effects. Beclomethasone is also available but there is less evidence for its use in COPD.
Combination inhaled corticosteroids with long-acting beta-2 agonists, e.g. budesonide with eformoterol
and fluticasone with salmeterol (fully subsidised under Special Authority), can be taken twice daily.
Theophylline, available in long-acting tablets or oral liquid, is used as a third-line treatment
for people with COPD when other bronchodilators are either ineffective or unavailable for long-term treatment.
Long-term continuous oxygen therapy (16 – 24 hours per day) may be of benefit in selected patients
with COPD who have a PaO2 consistently less than 55 mm Hg. Home oxygen is usually initiated by a respiratory physician.
The patient must be clinically stable and have stopped smoking for at least one month.
Inhaled anticholinergics and cardiovascular risk
A 2008 meta analysis assessing 17 trials found that inhaled anticholinergics were associated with a significantly
increased risk of cardiovascular death, myocardial infarction or stroke in people with COPD.18 However,
a 2010 study of almost 20 000 people with COPD found that tiotropium was associated with a reduction in cardiovascular
events and cardiovascular mortality.19 But another study, published in the same journal, showed an increased
risk of cardiovascular events associated with the use of ipratropium in a large population with COPD.20 There
is ongoing controversy regarding the interpretation of these results. There is currently no biological explanation
why the two anticholinergics would have different cardiovascular effects and a dose-response relationship for the effect
has not been demonstrated. Both medicines have low systemic absorption. Until the evidence is understood better, the
potential adverse effects of ipratropium and tiotropium need to balanced against the known benefits of these medicines.
Managing exacerbations
A COPD exacerbation is an acute event where symptoms deteriorate beyond normal day-to-day variation to the point where
a change in the medicine regimen is required.2 This is characterised by an increase in dyspnoea, cough or
sputum production, most commonly caused by a respiratory tract infection. COPD exacerbations are known to increase the
rate of lung function decline and are associated with increased rates of mortality.17 The higher rate of hospitalisation
amongst Māori and Pacific peoples due to COPD suggests that COPD exacerbations affect these groups more significantly
than other groups. People with COPD need to be able to identify exacerbations and seek treatment early.
Strategies to reduce the risk of exacerbations include:
- Improving exercise capacity
- Influenza vaccination (annually – funded for people with COPD) and pneumococcal vaccination (five-yearly) for people
with COPD and their families
- Reducing the risk of infection by avoiding people who have symptoms of an upper respiratory tract infection, e.g.
cough, rhinitis, nasal congestion, sneezing
- Avoiding smoke and other environmental pollutants, e.g. smog
- Optimised control of co-morbidities
- Warm and well ventilated homes
Treatment options for exacerbations include bronchodilation with SABA, either alone or in combination with short-acting
anticholinergics; doubling the dose or increasing the frequency of use, e.g. from four times to six times per day, if
necessary. If the patient is using a long-acting bronchodilator then this should also be continued during the exacerbation.
A short course of oral corticosteroids, e.g. 20 – 40 mg, once daily, for 7 – 14 days, may also reduce recovery time,
improve lung function and reduce the risk of a relapse.10
Antibiotics should only be used to treat exacerbations when there is an increase in cough, dyspnoea, sputum volume
or purulence. First-line treatment choice is amoxicillin 500 mg, three times a day, for five days. Second-line is doxycycline
100 mg, twice daily, for five days if the patient is penicillin allergic or has had a recent course of amoxicillin.
When a patient has a history of exacerbations, or may have difficulty accessing a general practice, a step-wise self-management
plan, including optimising bronchodilator use, oral corticosteroids and indications for antibiotic use may be useful.10
Referral to secondary care should be considered when:
- A previously mobile patient can no longer walk short distances
- Dyspnoea prevents eating or sleeping
- There is an inability to manage at home due to exhaustion
- A high-risk co-morbidity is present, e.g. heart failure or ischaemic heart disease
- There are sign of hypercapnia (CO2 retention) present, such as altered mental state
- There is an inadequate response to treatment or uncertain diagnosis
 For further information see: "Management
of acute exacerbations of COPD in Primary Care", BPJ 23 (Sept, 2009)
For further information see: "Management
of acute exacerbations of COPD in Primary Care", BPJ 23 (Sept, 2009)
Are Māori and Pacific peoples receiving optimal treatment for COPD?
Māori and Pacific peoples are three to four times more likely than people of other ethnicities to be hospitalised
due to COPD (Figure 1, page 15). Tiotropium is known to reduce COPD exacerbations and related hospitalisations.26 It
is available in New Zealand under Special Authority for patients with moderate or severe COPD.
Tiotropium dispensing rates in New Zealand by ethnicity (Figure 2) show that:
- Māori have a higher rate of tiotropium dispensing than other ethnicities, but are prescribed only one-third
to one-half more tiotropium despite hospitalisation rates being three to four times greater
- Pacific peoples have the same rate of hospitalisations for COPD as Māori, yet are prescribed less tiotropium
than Māori or European/Other people
It is unknown if these disparities are due to tiotropium not being prescribed to these patient groups, or if the
prescriptions are not being collected. In addition, there is no available data on medicine compliance for people with
COPD in New Zealand.
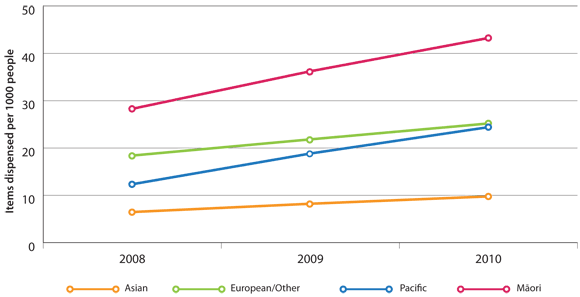
Figure 2: Age-standardised rates of tiotropium dispensing (items dispensed) in New Zealand per 1000
enrolled patients by ethnicity, 2008 to 2010*
* Discharges source: As per Figure 1. Population source: As per Figure 1
Pulmonary rehabilitation may improve symptoms of COPD
Pulmonary rehabilitation refers to programmes which combine multiple approaches to attempt to break the cycle of COPD,
where decreased physical activity due to dyspnoea leads to further loss of fitness and eventual immobility. There is
strong evidence that rehabilitation programmes improve the symptoms of COPD and reduce hospitalisations.10 Pulmonary
rehabilitation also reduces muscle wasting and weight loss, and programmes that include psychosocial support have been
associated with significant reductions in anxiety and depression.10,21 The minimum time-frame for a rehabilitation
programme to be beneficial appears to be six weeks,10 and the longer the programme lasts, the greater its
effectiveness.2 Family members play an important role in motivating a person with COPD to remain compliant
with their rehabilitation programme.
Weight loss is common in people with COPD as the added effort to breathe can increase energy requirements by 15–20%.22 People
with COPD who are underweight have increased mortality rates.22 Pulmocare is a high fat, low carbohydrate
dietary supplement, designed to minimise CO2 production. It is available under Special Authority for people with hypercapnia
as a result of COPD.
 For further information see: "The
nutritional management of unintentional weight loss in people with COPD", Prescription Foods (May, 2011).
For further information see: "The
nutritional management of unintentional weight loss in people with COPD", Prescription Foods (May, 2011).
Psychosocial support is particularly important for Māori and Pacific peoples with COPD. People with COPD have
an increased risk of developing symptoms of anxiety and depression, both of which are linked to poor health outcomes.10,23 In
addition, Māori and Pacific adults have a higher prevalence of mental health disorders in general than other ethnic
groups.24 Cognitive behavioural approaches have been shown to significantly reduce depression and improve
the health status of people with severe COPD.25 Strategies include relaxation, breathing techniques, positioning
and chest clearing techniques and modification of negative thoughts.25
 For further information on pulmonary rehabilitation programmes contact
the respiratory department at your local DHB. The Asthma Foundation's "Breathe easier with COPD" booklets provide a
list of suggested exercises that can be performed at home and practical ways of dealing with the stress and limitations
of COPD.
For further information on pulmonary rehabilitation programmes contact
the respiratory department at your local DHB. The Asthma Foundation's "Breathe easier with COPD" booklets provide a
list of suggested exercises that can be performed at home and practical ways of dealing with the stress and limitations
of COPD.
The Tu Kotahi Asthma Trust
Formed in the Hutt Valley in 1995, the Tu Kotahi Trust provides a Marae-based "by Māori, for Māori" support
programme for people with COPD. The goal of the group is to promote COPD education and a sense of togetherness and support
(whānaungatanga) throughout the whānau.
A research programme is currently underway to quantify the programme's outcomes. Anecdotal evidence from participants
indicates that the Trust is achieving success in providing timely access to health care for Māori affected by COPD.
The use of Te Reo Māori and understanding of culture (tikanga) and hospitality (manaakitanga) have created a non-threatening
and supportive environment for Māori. One participant described this by saying:
"...at the hospital they're speaking a language I could never understand, but you come here and sit down and use
language that I understand...that's a big barrier that got broken down...just getting to know on my level, instead
of me climbing up to theirs."
The group encourages participation in a regular exercise programme. Medicine compliance and management of exacerbations
have improved through the delivery of simple demonstrations and visual explanations. Members are also encouraged to
consider issues such as housing and co-morbidities when supporting people with COPD.
Through encouraging early engagement with individuals and their whānau, the Trust has identified a number of
people in their early 30s and 40s who are in the early phase of COPD. This is likely to provide significantly better
outcomes for these people, while simultaneously achieving financial savings through early disease management.
Raising community awareness and providing community-based resources for the diagnosis and management are important
strategies in combating COPD.
ACKNOWLEDGEMENT Thank you to Dr Matire Harwood, Clinical Director, Te Hononga O
Tamaki Me Hoturoa, Auckland, Dr Peter Martin, Medical Advisor, The Quit Group and Associate
Professor Jim Reid, Dunedin School of Medicine, University of Otago for expert guidance in developing this
article.