Successful treatment of long-term pain includes keeping patients active and engaged in their daily life.
Establish specific goals
Develop goals of care with the patient that are relevant to the patient and achievable, e.g. being able to walk the
dog by the next consultation.
Non-pharmacological approaches to reducing pain
Discuss “sleep hygiene” techniques with patients who have disturbed sleep. This emphasises reserving the bed and bedroom
for sleeping and sex. Advise patients to avoid watching television or using electronic devices in the bedroom and to leave
the room if they are awake for longer than 15 minutes, returning only when tired enough to sleep. Other advice includes
avoiding stimulants or diuretics, including alcohol close to bed-time and keeping a regular sleep routine, even during
weekends.
Tricyclic antidepressants, taken in the evening, may provide the dual benefit of pain relief and sedation overnight
(see: “The pharmacological treatment of neuropathic pain”).
 For further information on sleep hygiene, see: “Managing
insomnia”, BPJ 14 (Jun, 2008)
For further information on sleep hygiene, see: “Managing
insomnia”, BPJ 14 (Jun, 2008)
Staying active and engaged is a priority
If patients withdraw from their normal activities, discuss the reasons and consider whether any medicines require alteration.
Some patients may benefit from consultation with an occupational therapist, physiotherapist or counsellor. Patients with
low activity levels may benefit from supervised exercise or a Green Prescription.
Persistent pain is a strong risk factor for falls in older patients; exercise improves strength and balance.19 Improving
mobility to maintain independence and social participation will be a goal of care for many patients.
Planning can help patients fulfil their usual activities
People with long-term pain often tire quickly. Suggest patients devote time to important tasks early in the day or when
they have the most energy; this may led to a greater sense of control over their condition.
Support groups help patients connect and learn from others
Advice and information for patients about how to manage and live with long-term pain is available from:
Consider if support devices are necessary
Some patients may benefit from assistive devices, such as toilet and shower rails. Occupational therapists may be able
to organise subsidised installations of these supports for select patients. Patients at risk of falls may benefit from
a medical alarm.
Other non-pharmacological approaches have limited evidence of efficacy
Interventions such as acupuncture or transcutaneous electrical nerve stimulation (TENS) may be useful for patients who
have ongoing pain despite medication.20 There have been few clinical trials, however, assessing their efficacy
or comparing their effects with pharmacological treatments.
Psychological approaches, such as cognitive-behavioural therapy (CBT), may help patients adapt to living with pain,
however, there is little evidence to suggest they reduce neuropathic pain.
The pharmacological treatment of neuropathic pain
The medicines recommended for the initial treatment of neuropathic pain include (see Table 2 for
dosing):1, 21
- TCAs, such as amitriptyline and nortriptyline – fully subsidised, unapproved indication. Amitriptyline is the TCA
with the strongest evidence of effectiveness and is favoured in some international guidelines.1, 21 However,
nortriptyline has less anticholinergic activity than amitriptyline and less adverse effects in elderly patients.22,
23
- Gabapentin – fully subsidised with Special Authority approval for patients diagnosed with neuropathic pain.
The related medicine pregabalin has a similar efficacy and adverse effect profile as gabapentin, and is available
unsubsidised. Be mindful that co-administration of morphine can increase levels of gabapentin and dosing
may need to be adjusted.24
- Carbamazepine – initial treatment for patients with trigeminal neuralgia or diabetic polyneuropathy; fully subsidised.
These medicines have evidence of efficacy and are first or second-line treatments for neuropathic pain;21, 25 guidelines
do not favour one initial medicine over another.1, 21 For most neuropathic pain medicine efficacy is not dependent
on the underlying cause, therefore, potential adverse effects may dictate the choice of treatment, e.g. patients with
central neuropathic pain may be less tolerant of medicines with CNS adverse effects.21 The patient’s need for
analgesia may fluctuate and regular follow-up is important. For patients with central-post stroke pain there is evidence
that medicines are less effective.26
Tricyclic antidepressants, gabapentin, either alone or in combination, and carbamazepine (for trigeminal neuralgia and
diabetic neuropathy) are appropriate options for treating most types of neuropathic pain in primary care. Alternative
anticonvulsant medicines, e.g. valproate or lamotrigine may be trialled in primary care, however, there is limited evidence
of effectiveness in patients with neuropathic pain.
The role of non-steroidal anti-inflammatory drugs (NSAIDs) is unclear. NSAIDs are not included as treatment
options in neuropathic pain guidelines.1, 27 However, there is insufficient evidence to conclude that they
are ineffective, and in practice NSAIDs are often used by patients with neuropathic pain.28 It is possible
that NSAIDs have an effect in patients with mild pain or pain with an inflammatory component or they may have a placebo
effect in some patients.28
Other antidepressants have a limited role in the treatment of neuropathic pain. TCAs are preferred
over selective noradrenaline reuptake inhibitors (SNRIs) or selective serotonin reuptake inhibitors (SSRIs) for patients
with neuropathic pain.
Venlafaxine is the only SNRI available in New Zealand which has evidence of efficacy in the treatment of neuropathic
pain.29 Consultation with a neurologist or clinician in a pain clinic is recommended before using venlafaxine
as a treatment for patients who have not responded sufficiently to initial treatment. Duloxetine is recommended as a potential
first-line treatment for neuropathic pain by international guidelines,1 however, this medicine is not available
in New Zealand.
There is currently no evidence that SSRIs are effective in the treatment of neuropathic pain. 21
Opioids are reserved for patients with severe neuropathic pain due to the potential adverse effects,
including dependency.2, 21 Discussion with a clinician experienced in treating pain is recommended before prescribing
opioid-based medicines for patients with neuropathic pain that is not controlled by other approaches.
Topical treatment of cutaneous neuropathic pain
Topical capsaicin cream (0.075%) is a treatment option for patients with localised, cutaneous neuropathic pain.21 It
is fully subsidised by prescription endorsement for patients diagnosed with post-herpetic neuralgia or diabetic peripheral
neuropathy. Capsaicin cream produces a burning sensation on application; patients should wash hands immediately after
application and avoid transferring the product to eyes or mucous membranes.29
Lidocaine 2.5% + prilocaine 2.5% patches (unsubsidised) may be a second-line topical analgesic for patients who do not
tolerate capsaicin cream.21
Initiating and optimising treatment
For most analgesics, dosing should start low and be titrated upwards. Practice points for optimising treatment for neuropathic
pain are shown in Figure 1. Treatment success is measured by subjective assessment of the patient’s level of pain, sleep
disruption and ability to function in daily life.
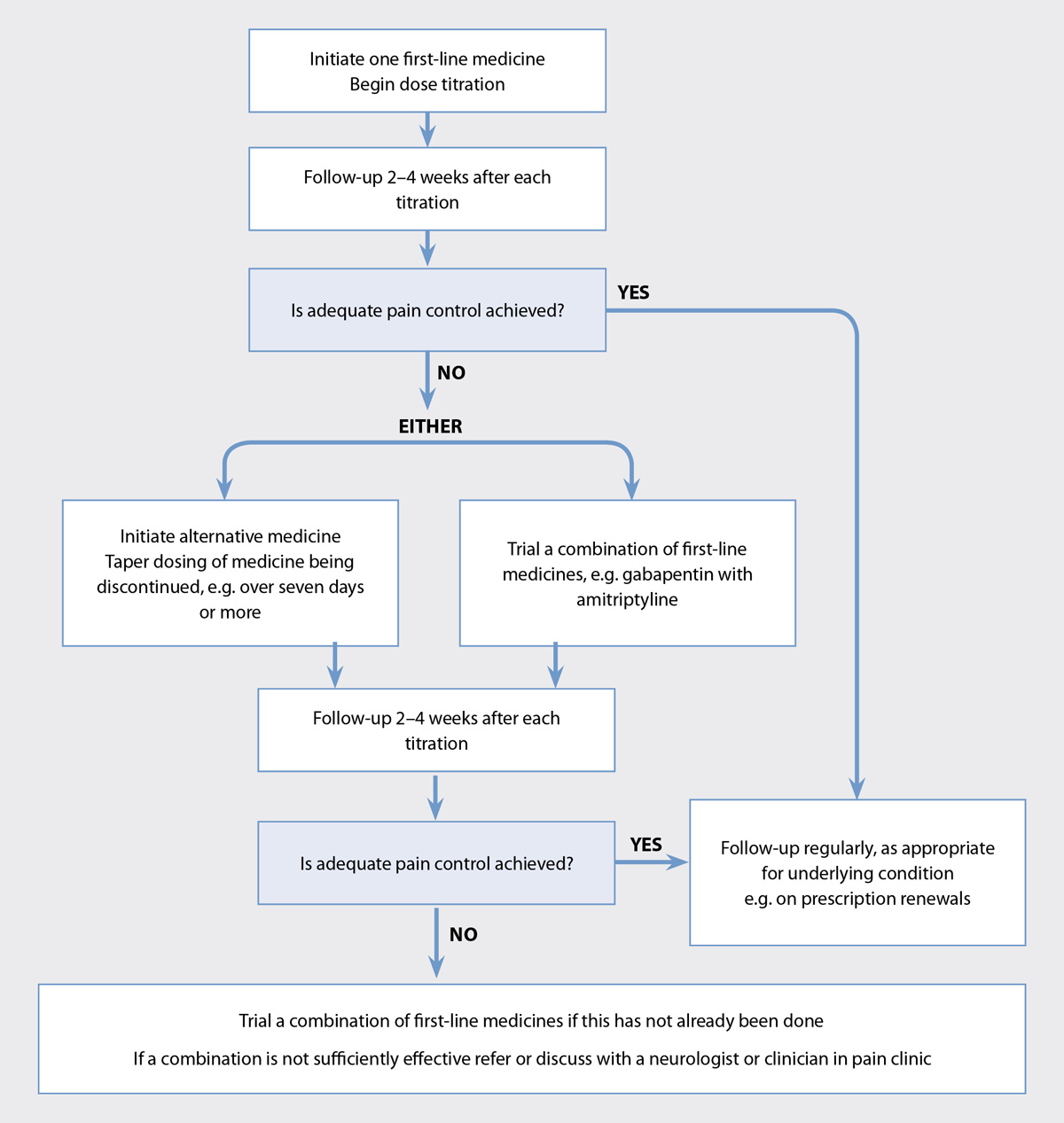
Figure 1. Optimising analgesia for neuropathic pain, except trigeminal neuralgia.1, 5
If sufficient control is not obtained with the first medicine trialled, switch to another of the recommended
initial medicines, e.g. from a TCA to gabapentin, or use a combination of medicines. If medicines are switched, gradually
reduce the dose of the medicine being withdrawn, e.g. over seven days or more, while the new medicine is initiated in
order to provide continuous treatment. If medicines are combined, aim for a combination of medicines that have different
analgesic mechanisms.30 Combination treatment with a TCA and gabapentin provides better outcomes, at lower
doses, without additional adverse effects, than either of these medicines alone in higher doses. For example, nortriptyline
50 mg, daily, with gabapentin 2000 mg, daily in divided doses, provided better pain relief in one study than nortriptyline
60 mg, daily or gabapentin 2250 mg, daily.30
Treating trigeminal neuralgia
Carbamazepine should be the initial treatment trialled for patients with trigeminal neuralgia.1 If carbamazepine
is ineffective, consider consulting with a neurologist or pain clinic as there is little evidence from clinical trials
to guide prescribing of other medicines.1, 31 Lamotrigine or baclofen (unapproved indications) have been suggested
as potential second-line treatment options for patients with trigeminal neuralgia.31
 Best practice tip: Consider strategies for reducing “piles of
pills” for patients with long-term pain, e.g. more frequent dispensings during an analgesic trial. Patients with chronic
pain may have comorbidities and be taking a number of medicines; consider whether they are eligible for the Long Term
Condition service to simplify their medicine regimen.
Best practice tip: Consider strategies for reducing “piles of
pills” for patients with long-term pain, e.g. more frequent dispensings during an analgesic trial. Patients with chronic
pain may have comorbidities and be taking a number of medicines; consider whether they are eligible for the Long Term
Condition service to simplify their medicine regimen.
 For further information, see: “Piles of pills: Prescribing appropriate
quantities of medicines”, BPJ 69 (Aug, 2015).
For further information, see: “Piles of pills: Prescribing appropriate
quantities of medicines”, BPJ 69 (Aug, 2015).