The volume of oxycodone prescribed in New Zealand is continuing to rise, despite efforts to encourage clinicians to use this medicine appropriately. Approximately 30% of oxycodone is initiated within general practice. A further 17% of prescriptions are continued by General Practitioners, when initiated outside general practice. Knowledge of a patient’s clinical and medicines history and psychosocial background puts General Practitioners in a strong position to not simply “go with the flow”, but instead re-evaluate the indication for oxycodone.
In this article

View / Download pdf version of this article
Update: Oxycodone: how did we get here and how do we fix it? (BPJ62)
Approximately 70% of people dispensed oxycodone in New Zealand are initiated on this medicine outside
of general practice, i.e. by a doctor in secondary care. This supports the claim that much of the use of
oxycodone is driven by secondary care prescribing. However, 30% of all prescriptions for oxycodone are
initiated by a General Practitioner. In addition 17% of patients initiated on oxycodone in secondary care
have their prescriptions continued by a General Practitioner. Oxycodone is a strong opioid indicated for
the treatment of moderate to severe pain, when morphine is not tolerated, and all other options have
been considered. Clinicians are urged to assess whether oxycodone is appropriate whenever initiating or
continuing a prescription for this medicine.
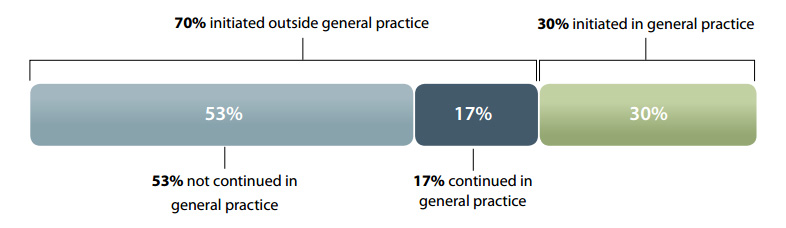
Figure 1: Source of prescriptions for patients initiated on oxycodone in 2011 (Pharmaceutical Warehouse dispensings)
Why is oxycodone a problem?
Oxycodone is not a new medicine. It was first synthesised
in 1916 in Germany and became available for clinical use
in the United States by 1939. For many years it has been
used overseas as a component in combination short-acting
analgesics. A controlled release formulation of oxycodone
alone was released in the United States in 1996 and was in New
Zealand by 2005. Since then, use of this medicine has increased
dramatically and many countries are now dealing with issues
of misuse, addiction and illegal diversion of prescriptions.
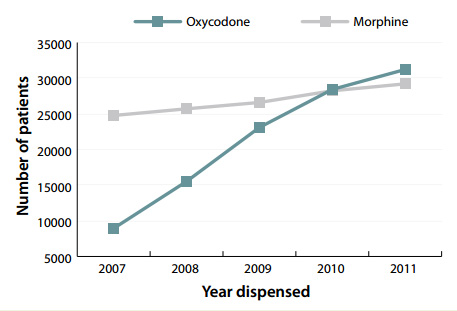
Figure 2: Number of patients dispensed oxycodone and morphine 2007-11 (Pharmaceutical Warehouse dispensings)
In New Zealand, the use of oxycodone has increased by
249% over the last five years (Figure 2). This has not been
accompanied by a corresponding decrease in prescriptions for
morphine, and the total amount of strong opioids dispensed
is climbing rapidly.
This raises several questions:
- Which patients are being prescribed oxycodone? And by
whom?
- Has the marketing of oxycodone been so effective that
a whole new group of patients now “require” strong
opioids?
- Is oxycodone being inappropriately prescribed instead of
analgesics that are lower on the WHO pain ladder? If so,
why?
We encourage every clinician to look critically at their
prescribing of oxycodone and, if necessary, make changes
on how they prescribe this medicine.
What is the appropriate indication for oxycodone?
There is no dispute that oxycodone is an effective analgesic,
however, prescribing figures suggest that it is being chosen as
the first-line opioid in many situations when it should not be.
Morphine is the preferred first-line option for the treatment
of acute and chronic moderate to severe pain, when a strong
opioid is indicated. When compared to morphine, oxycodone:
- Has no better analgesic efficacy
- Has a similar adverse effect profile
- May have more addictive potential1,2
- Is significantly more expensive
Oxycodone should only be prescribed for the treatment
of moderate to severe pain in patients who are intolerant
to morphine and when a strong opioid is the best option.
Although oxycodone has been reported to be potentially
safer than morphine in patients with renal impairment, active
metabolites can still accumulate.3 Fentanyl or methadone are
likely to be safer in patients with renal impairment, who require
a strong opioid, because they have no clinically significant
active metabolites.3 Discussion with a pain or renal physician is
recommended when considering the use of any strong opioid
in a patient with severe renal impairment (creatinine clearance
< 30 mL/min).
 For further information see:
For further information see:
Oxycodone misuse in New Zealand
The Illicit Drug Monitoring System (IDMS) provides
surveillance on the misuse of drugs in New Zealand.
Oxycodone was first noted as an emerging drug of
misuse by the IDMS in 2008. The latest report (to the
end of 2010) shows that oxycodone is continuing to
feature prominently amongst people who misuse
drugs. Oxycodone was the second most common new
drug to be used in 2010 by methamphetamine users,
behind synthetic cannabis (which is now unavailable for
commercial sale). In 2010, 18% of injecting drug users had
illicitly used oxycodone in the past six months, compared
to 9% in 2008.4 Pharmaceutical morphine remains one
of the principal opioids used by injecting drug users in
New Zealand (along with “homebake” heroin/morphine
and methadone).4 The available supply of diverted
opioids is directly related to the total amount of opioids
prescribed.5
Although other controlled release opioids can also be
tampered with, the controlled release form of oxycodone
(OxyContin), is rapidly gaining popularity as a drug of
misuse. There has been criticism that the information
warning patients not to break, chew or crush the tablets
to avoid rapid release and absorption of a potentially
harmful dose of oxycodone, may have actually instructed
people in how to misuse the medicine.6,19 In response
to this problem in the United States and Canada, the
controlled release formulation has been replaced by a
newer extended release formulation (OxyNeo) aimed to
be tamper-resistant.7,8 In Canada from 2013, a special
application will be required for patients to access
oxycodone, unless they are being treated for cancer pain
or palliative care.8 No changes to prescribing regulations
or medicine formulation have been announced for New
Zealand or Australia.
What can General Practitioners do to reduce
oxycodone use?
Data from the Pharmaceutical Warehouse show that 30%
of prescriptions for oxycodone are initiated within general
practice (Figure 1). When considering initiation of oxycodone,
always ask yourself if you would use morphine for this patient. If
the answer is no then do not prescribe oxycodone. Oxycodone
should not be prescribed when a weaker opioid, e.g. codeine,
dihydrocodeine or tramadol, would be more appropriate.
Remember that: 5 mg oxycodone is approximately equivalent
to 10 mg morphine, 50 - 100 mg tramadol, 100 mg
dihydrocodeine or 100 mg codeine.9,10
 Best Practice Tip: Make it a practice policy, whenever
prescribing a strong opioid, to record why the patient has been
prescribed this medicine, the usual dose, the expected time
frame for treatment, any concerns regarding the patient (such
as low mood, poor social support) and specific instructions
regarding actions if an increased dose is requested, an early
prescription is sought, or if medicines are reported as lost.
Best Practice Tip: Make it a practice policy, whenever
prescribing a strong opioid, to record why the patient has been
prescribed this medicine, the usual dose, the expected time
frame for treatment, any concerns regarding the patient (such
as low mood, poor social support) and specific instructions
regarding actions if an increased dose is requested, an early
prescription is sought, or if medicines are reported as lost.
Patients on oxycodone initiated in secondary care
Approximately 70% of oxycodone is initiated within secondary
care. Prescribing data show that when oxycodone is initiated
from outside general practice, 17% of patients have their
prescription continued by a General Practitioner (Figure 1).
Knowledge of a patient’s clinical and medicines history and
psychosocial background puts General Practitioners in a
strong position to not simply “go with the flow”, but instead
re-evaluate the indication for oxycodone, even if it has been
initiated within secondary care.
Summary: management strategies for patients
discharged on oxycodone
When a patient is discharged from secondary care on
oxycodone, a suggested management strategy is as
follows:
- When the patient presents for a renewal of a
prescription of oxycodone, assess their level of
pain and consider whether a strong opioid is
still required.
- If a strong opioid is no longer required, step
down to a weaker opioid or to paracetamol.
Depending on the length of time the patient
has been on oxycodone, a gradual tapering of
the dose may be necessary.
- If a strong opioid is still required, consider
changing the patient to morphine. Explain to
the patient that morphine is equally effective,
will not usually result in any other adverse
effects and that it is the preferred option when
strong opioids are used in general practice.
Regularly reassess the patient and step-down
treatment as appropriate.
Make sure the patient knows that oxycodone is a strong
opioid
Many patients are unaware (and shocked to be told) that
oxycodone is a strong opioid similar to morphine, but milligram
for milligram, twice as potent. Both patients and clinicians
have been known to mistakenly associate oxycodone with the
weak opioid codeine, rather than with morphine, because of
the similarity in the names of the medicines.
Reassess why oxycodone was initially prescribed
Establish the precise clinical problem for which oxycodone
was initially prescribed, e.g. post-surgical pain or an acute
injury. Does this same problem exist now? Most patients can
gradually reduce analgesia in the days to weeks after surgery
or acute injury.
What level of pain is the patient experiencing?
If there is an ongoing medical condition that requires analgesia,
check that the level of pain being experienced warrants the
use of a strong opioid.
Consider if oxycodone can be stopped
If the pain has reduced and oxycodone is no longer required,
stop or taper the dose (next section). Weaker analgesia, such as
codeine and paracetamol, may still be required. Tramadol
and dihydrocodeine can also be used as alternatives. Check
the patient’s understanding of any analgesic medicines that
are used - are they being taken at the right time and in the
right dose to gain effective pain relief and to minimise adverse
effects?
Consider switching the patient to morphine
If a strong opioid analgesic is still indicated, consider switching
the patient to morphine. Morphine should be the strong opioid
of choice for the majority of patients unless they are allergic to
morphine or intolerant to its adverse effects. A dose of 5 mg
of controlled release oxycodone is approximately equivalent
to 10 mg of long-acting morphine. This conversion rate is,
however, only approximate and there is varying guidance on
the dose of morphine that should be used when switching.9,10
If the aim is to eventually discontinue opioids and the degree
of pain allows, calculate the equivalent dose of morphine and
then start the patient on half of this dose.2 The response of
the patient to the change in medicine should be reviewed
regularly and the dose adjusted as required to prevent any
withdrawal symptoms. The “ABC” of opioid pain medicine use
should be remembered:
- Anti-emetic prescription if nausea present
- Breakthrough dose of morphine may be required
- Constipation is likely, prescribe a laxative
Detecting aberrant drug taking
behaviour
Behaviours that may suggest the development of
aberrant drug taking behaviour, such as overuse, hoarding,
dependence and diversion, include: presenting early for
repeats, loss of prescriptions or medicines or requests for
an escalation in dose.
Patients with chronic pain who take opioid medicines
may over time become tolerant or dependent and require
increased doses to enable them to function day to day.6 If
the patient reports that their pain is worsening, consider if
this would normally be expected with the condition being
treated, if a different diagnosis should be considered or
whether there is the possibility of misuse.
Addiction to opioids is reported to occur in only a small
number of patients with chronic pain. However, many
more patients with chronic pain display aberrant drug
taking behaviour.12,13
Personal or family history of alcohol or drug dependence
increases the risk of misuse of opioids. The presence of
an anxiety disorder or depression further increases this
risk.14,15 However, patients who misuse medicines do not
always fit a stereotype and risk factors may not always
be apparent. Any person, regardless of gender, age,
ethnicity, income, health or employment status can be
at risk of aberrant drug taking behaviour. It is therefore
recommended that every patient who is prescribed
an opioid is assessed for risk factors for aberrant drug
taking behaviour, including the possibility of diversion of
prescriptions.
If an opioid is continued, establish a pattern of regular
review
Every patient prescribed a strong opioid analgesic on an
ongoing basis requires regular review. The requirement
for monthly prescriptions for opioids provides an ideal
opportunity to review the need for the medicine, however, in
some situations review will need to be more frequent, such as
early in the course of treatment. Discuss the dose, the goals
of treatment, adverse effects, the time frame for the use of
opioid and if appropriate develop a clear plan for stopping the
medicine. Check with the patient how they are managing day
to day. The Australian and New Zealand College of Anaesthetists
recommends a “5A assessment” when prescribing a strong
opioid: assess the patient’s analgesia, activity, adverse effects,
affect and aberrant drug taking behaviour (see “Detecting
aberrant drug-taking behaviour”).11 Referral to a specialist
pain clinic may be required if the patient’s pain is unable to
be effectively controlled or if there are other concerns with
aspects of the “5A” assessment.
How to discontinue oxycodone
Abrupt cessation
Patients who have been taking oxycodone at low doses
(e.g. 10 - 20 mg daily) for less than one to two weeks
can generally stop the medicine without experiencing
withdrawal symptoms.16 Gradual tapering of oxycodone to
avoid withdrawal symptoms is recommended in most other
situations.
Gradual dose reduction
Patients who have been taking oxycodone for more than one
to two weeks, or at high doses, should have the dose gradually
tapered to avoid symptoms of opioid withdrawal.2,6
How quickly and by how much the oxycodone can be reduced
will depend on the current dose, the length of time the
medicine has been taken for and individual patient factors,
such as anxiety, co-morbidities (e.g. depression or other
psychiatric conditions) and the likelihood that the patient is
dependent on oxycodone, in which case the dose should be
reduced more slowly.2,6
Advice about tapering of opioids varies widely in the literature,
however, in general: 2,6,16
Reduce the dose in 20-25% increments or, if required,
more slowly by 5-10%
Reductions can be made every two or three days
Once the patient has been reduced to one-third of the
initial dose, the rate of taper should be slowed
Consider holding the dose at the same level if the patient
develops withdrawal symptoms, an increase in pain or
lowered mood
Most patients can be withdrawn from oxycodone within
one month, depending on how high the dose was prior
to initiating tapering
Referral to addiction services
In some situations it may be more appropriate to refer patients
to a community based drug and alcohol programme, to
withdraw from oxycodone. Patients who may benefit from
referral include those who:17
- Are unable to be slowly tapered off oxycodone in general
practice due to factors such as a lack of success with
tapering, non-compliance with tapering, accessing
opioids from other sources
- Are misusing oxycodone or other addictive substances
(including alcohol)
Opioid withdrawal symptoms
Abrupt cessation of any strong opioid can produce
extremely unpleasant and distressing withdrawal
symptoms, depending on the dose and the length of
time the medicine has been used for.18 These symptoms
reach a peak approximately three days after the opioid
is stopped and may last for approximately 7-10 days.19
Although opioid withdrawal is very unpleasant for the
patient, it is not usually associated with a risk of seizure
or delirium, unlike abrupt cessation of such substances as
alcohol or benzodiazepines.18,19
Opioid withdrawal symptoms can include insomnia,
dysphoria, yawning, rhinorrhoea, piloerection,
perspiration, lacrimation, tremors, restlessness, poor sleep,
nausea or vomiting, diarrhoea, muscle aches and twitches,
abdominal cramps, anxiety and an increase in pain.6,16
If required, medicines that may assist with the treatment
of withdrawal symptoms include:
- Clonidine which decreases adrenergic activity
and may relieve symptoms such as nausea,
sweating, cramps and tachycardia: oral dose 50-75
micrograms up to three times a day, or alternatively
a transdermal patch may be used if there are
concerns about adherence to oral dose
- A sedating antihistamine may help if the patient is
restless and unable to sleep
The role of strong opioids for chronic non-cancer pain
The use of strong opioids for chronic non-cancer pain is controversial and there is limited quality evidence to support or oppose their use for this type of pain.11,12 Principles for the use of opioid analgesics in people with chronic non-cancer pain have been developed by the Australian and New Zealand College of Anaesthetists.11 The principles aim to take into account both the widely varying individual response to opioids and the risks for an individual patient. The use of opioids for chronic non-cancer pain should be regarded as an “ongoing individual trial of therapy”.11
Assess all aspects of the pain
Consider factors that may influence the nature and intensity of pain and the patient’s reaction to the pain. Ask about the patient’s beliefs about the underlying problem, their mood, their fears and their expectations of pain treatment. Discuss the goals of treatment with the patient - a reduction in pain and an increase in function are realistic and achievable outcomes, while an expectation that the pain will be totally eliminated may be unrealistic.17,20
Pain can be difficult to assess because it is subjective and is often influenced by factors such as mood, stress and the psychosocial support that the patient has. The most clinically useful pain scales include an assessment of the impact of the pain on daily life. Pain can have a significant effect on daily activities, e.g. altering sleep or appetite. It can induce or exacerbate depression and anxiety, it can influence social interactions, prevent work and impair relationships.
 For further information about pain scales, see “Pharmacological management of chronic pain”, BPJ 16 (Sep, 2008).
For further information about pain scales, see “Pharmacological management of chronic pain”, BPJ 16 (Sep, 2008).
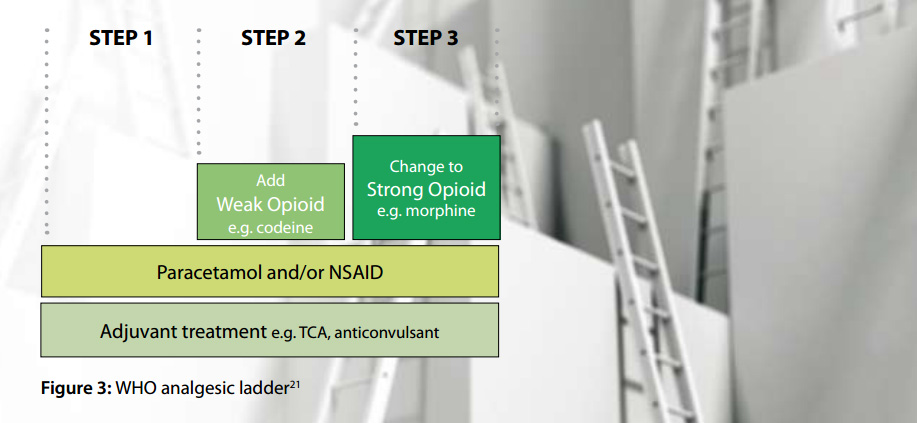
Ensure there has been an adequate trial of other treatments
The WHO analgesic ladder provides a step-wise approach to analgesia for the management of pain (Figure 3).21 Adjuvant treatments such as tricyclic antidepressants and anticonvulsants, can be included at every step of the ladder, especially for patients with neuropathic pain, and it is recommended that they are considered before the use of strong opioids, i.e. Step 3.11 Non-pharmacological treatment of pain is also important. This includes ensuring that the patient understands the underlying problem and the treatment plan, checking on family and social supports, promoting the benefit of healthy lifestyle choices (e.g. exercise, adequate sleep, balanced diet) and the involvement of other health professionals, e.g. physiotherapist, occupational therapist, psychologist, pain clinic specialist.
Consider if a strong opioid is indicated and appropriate for the patient
Prior to initiating a strong opioid for chronic pain in particular, consider the following questions:
- Have I identified the cause of the pain?
- What am I trying to achieve?
- Is this what the patient wants?
- To what extent are psychosocial factors contributing to the pain level and how can these factors be addressed?
- Is there evidence that a particular medicine will help this type of pain?
- Are there non-pharmacological alternatives?
- Do the potential benefits outweigh the harms of the treatment? Check if the patient has a history of addictive behaviour, alcohol or medicine misuse. If the patient has a current or past history of a psychological problem, a strong opioid may not be appropriate.
- Have I provided effective education about the most appropriate way to use analgesics?
- Have I considered how long a strong opioid may be required for?
- Have I made a plan for follow up?
Reach an agreement with the patient regarding a trial of strong opioid analgesic
If a strong opioid is indicated, ensure the patient has a good understanding of the type of medicine to be used and the goals of treatment, i.e. an increase in function rather than complete resolution of pain. The patient should be made aware of the potential problems with strong opioids, including adverse effects, safety issues and the potential for dependency and misuse. It is also recommended that an agreement is reached so that if the goals are not achieved, adverse effects are intolerable or there are concerns about misuse, the opioid will be discontinued.11,20 Any agreement should be clearly documented in the patient notes. This should include guidance about management if the patient requests or presents for an early repeat, if the medicine is reported as lost or there is a request for an increase in dose. When a strong opioid is prescribed, ideally there should be one prescriber and one pharmacy involved.
Start with an appropriate dose and slowly titrate as required
Choose a low starting dose of a long-acting or extended release preparation of a strong opioid, usually morphine as the first-line choice. Most patients taking opioids will also require a laxative, and possibly an anti-emetic (in the initial stages of treatment), as well as short-acting medicine for breakthrough pain. It is recommended that the dose be slowly titrated over several weeks if required, with a clinical assessment prior to each increase in dose. The Australian and New Zealand College of Anaesthetists recommends a “5A” assessment which includes a review of:11
- Analgesia
- Activity
- Adverse effects
- Affect
- Aberrant behaviour
A suggested time frame for a trial of a strong opioid is four to six weeks.
9 If the treatment has been of no benefit after this time, the dose of the opioid should be tapered and then stopped.
Regularly review the patient
Once the patient is established on an effective dose, regularly reassess them using the “5A” assessment. Check that the goals of treatment agreed initially are being achieved and that a strong opioid is still the most appropriate medicine for the patient. If the patient requests an increase in dose consider whether this may reflect:
- A change in the underlying condition producing pain
- The patient’s current mood, life stressors or other social circumstances
- The development of tolerance
- Opioid induced hyperalgesia (abnormal sensitivity to pain due to prolonged use of strong opioids)20
- Aberrant drug taking behaviour
ACKNOWLEDGEMENT Thank you to Dr Geoff Robinson, Chief Medical Officer, Addiction Medicine Specialist, Capital & Coast DHB and Dr Howard Wilson, General Practitioner and Pharmacologist, Canterbury, members of the analgesic subcommittee of the Pharmacology and Therapeutics Advisory Committee to PHARMAC for expert guidance in developing this article.
References
- Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharm 2008;196:105-16.
- Kahan M, Mailis-Gagnon A, Wilson L, Srivastava A. Canadian guideline for safe and effective use of opioids for chronic noncancer pain. Clinical summary for family physicians. Part 1: general population. Can Fam Physician 2011;57:1257-66.
- King S, Forbes K, Hanks GW, et al. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: A European Palliative Care Research Collaborative opioid guidelines project. Palliat Med 2011;25(5):525-52.
- Wilkins C, Sweetsur P, Smart B, Griffiths R. Recent trends in illegal drug use in New Zealand, 2006-2010. Findings from the 2006, 2007, 2008, 2009 and 2010 Illicit Drug Monitoring System (IDMS). Auckland: Social and Health Outcomes Research and Evaluation (SHORE), School of Public Health, Massey University, 2010.
- Wilkins C, Sweetsur P, Griffiths R. Recent trends in pharmaceutical drug use among frequent injecting drug users, frequent methamphetamine users and frequent ecstasy users in New Zealand, 2006-2009. Drug and Alcohol Review 2011;30:255-63.
- Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care Clin Offic Pract 2011;38:71-90.
- U.S. Food and Drug Administration (FDA). FDA approves new formulation for OxyContin. FDA news release. 5th April 2010. Available from: www.fda.gov (Accessed May 2012).
- Ministry of Health and Long-term Care. Change in funding status of oxycodone controlled release tablet (discontinuation of OxyContin and introduction of OxyNEO). Available from: www.health.gov.on.ca/en/public/programs/drugs/ons/oxy_faq.aspx (Accessed May, 2012).
- Australian Medicines Handbook Adelaide: Australian Medicines Handbook Pty Ltd, 2011.
- British National Formulary (BNF) 62. London: Pharmaceutical Press, 2011.
- Faculty of Pain Medicine. Australian and New Zealand College of Anaesthetists. 2010. Principles regarding the use of opioid analgesics in patient with chronic non-cancer pain. Available from: www.fpm.anzca.edu.au (Accessed May, 2012).
- Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician 2010;13:401-35.
- Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic non-malignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviours? A structured evidence-based review. Pain Med 2008;9(4):444-59.
- Monheit B. Prescription drug misuse. Aust Fam Physician 2010;39(8):540-6.
- Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug Alcohol Rev 2011;30:300-5.
- Gordon D, Dahl J. Opioid withdrawal, #95, 2nd edition. J Pall Med 2011;14(8):965-6.
- Kahan M, Wilson L, Mailis-Gagnon, Srivastava A. Canadian guideline for safe and effective use of opioids for chronic noncancer pain. Clinical summary for family physicians. Part 2:special populations. Can Fam Physician 2011;57:1269-76.
- Chou R, Fanciullo GJ, Fine PG et. al. Opioid treatment guidelines. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10(2):113-30.
- Department of Health and Community Services, Newfoundland and Labrador. Oxycontin Task Force, Final report. June 30, 2004. Available from: www.health.gov.nl.ca/health/publications/oxycontin_final_report.pdf (Accessed May, 2012).
- British Pain Society. Opioids for persistent pain: good practice. A consensus statement prepared on behalf of the British Pain Society, the Faculty of Pain Medicine of the Royal College of Anaesthetists, the Royal College of General Practitioners and the Faculty of Addictions of the Royal College of Psychiatrists. January 2010. Available from: www.britishpainsociety.org (Accessed May, 2012).
- WHO analgesic ladder. Available from: www.who.int/cancer/palliative/painladder/en/ (Accessed May, 2012).