Dabigatran – practical considerations for General Practitioners
 For revised information on the use of dabigatran, see "Dabigatran revisited", BPJ 50 (February, 2013).
For revised information on the use of dabigatran, see "Dabigatran revisited", BPJ 50 (February, 2013).
Dabigatran (Pradaxa) is now available in New Zealand, fully funded, without Special Authority, as an alternative oral
anticoagulant to warfarin, to prevent stroke in people with non-valvular atrial fibrillation (AF). Dabigatran is also
registered for short-term use for the prevention of venous thromboembolism (VTE) after major orthopaedic surgery. It is
available in 75 mg, 110 mg and 150 mg capsules.
Dabigatran is the first new oral anticoagulant that has been made available for clinical use for more than fifty years.
It was approved for use in AF in October 2010 in the United States and Canada, and in 2011 in Japan and some European
countries. Although it has been used since 2008 for short term prophylaxis of VTE, clinical experience in the “real
world” setting is still limited and data on longer term safety is lacking. Recommendations for its use in AF are
based largely on the Randomised Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial (see below for further
discussion on this trial).1
Warfarin has a history of many years of clinical use but has two major limitations – a narrow therapeutic range of safe
anticoagulation and a highly variable dose response. Variation may also occur for individual patients over time due to
interactions with certain dietary components and the use of other medicines. Laboratory monitoring with INR and dose adjustment
is required to achieve individually tailored, adequate, safe anticoagulation. In contrast, dabigatran has a predictable
effect on anticoagulation and therefore routine monitoring is unnecessary. For this reason, dabigatran is likely to be
more convenient than warfarin, however, it requires twice daily dosing. Dabigatran appears to be at least as effective
as warfarin for preventing stroke in patients with AF, and has similar rates of bleeding (see below for a
discussion of the evidence).

What are the registered indications for dabigatran?
Dabigatran is indicated for people with non-valvular atrial fibrillation for:2
- Prevention of stroke
- Prevention of systemic embolism
- Reduction of vascular mortality
Treatment should be continued life-long unless the risk benefit ratio for the patient changes.
Dabigatran is also registered for short term use for the prevention of venous thromboembolism (VTE) after major orthopaedic
surgery.2 It therefore provides an oral alternative to low molecular weight heparin, e.g. enoxaparin.
What should dabigatran not be used for?
There has, as yet, been no research on the use of dabigatran in people with AF who have haemodynamically significant
valvular heart disease or in people with artificial valves.1,3
Dabigatran should not be used for patients who require long-term prophylaxis for deep venous thrombosis or pulmonary
embolism. Trials are underway to determine the effectiveness of dabigatran for long-term prophylaxis. It is not known
whether dabigatran is clinically effective for VTE prophylaxis for long haul flights.
There have also been no studies investigating the use of dabigatran in people aged under 18 years or in pregnant women.2 Clinical
data on the excretion of dabigatran into breast milk is not available.2
How does dabigatran work?
Dabigatran etexilate, a direct thrombin inhibitor, is a prodrug (a medicine administered in an inactive form) which
is converted to the active medicine dabigatran after oral administration.2 Conversion to the active form takes
place rapidly in the plasma and liver and an effective anticoagulant effect can be attained within two to three hours
of oral ingestion.2,4 It takes two to three days to reach steady state.5
The active form, dabigatran, is a potent, competitive and reversible (in vitro) direct inhibitor of the active site
of thrombin (factor IIa).2,6 It has high affinity and specificity for thrombin. Warfarin, in contrast, produces
its anticoagulant effect via activity on a number of different coagulation factors (see below - “How
do warfarin and dabigatran affect coagulation?”). The anticoagulant effect of dabigatran therefore, has been
shown to be predictable and consistent with a wide therapeutic window which allows for a fixed dose regimen.2,4
What are the recommended doses of dabigatran?
For the prevention of stroke in people with non-valvular atrial fibrillation the recommended dose of dabigatran is:2
- 150 mg, twice daily, for patients with a creatinine
clearance >30 mL/min*
- 110 mg, twice daily, for patients aged ≥ 80 years (because of the likelihood of an age-related decline in renal function)
For VTE prophylaxis following major orthopaedic surgery the recommended dose of dabigatran is:2
- 220 mg (2 × 110 mg tablets), once daily, for patients with creatinine clearance > 50 mL/min
- 150 mg ( 2 × 75 mg tablets), once daily, for patients with creatinine clearance 30 – 50 mL/min
N.B. The length of the course varies with the type of surgery – knee replacement surgery ten days, hip replacement surgery
35 days.
Dabigatran is predominately renally excreted
Renal excretion is the dominant elimination pathway for dabigatran. Up to 80% of circulating unchanged dabigatran and
small amounts of dabigatran glucuronides are excreted via the kidneys.2 Consequently, a reduction in renal
function results in elevated plasma concentrations of dabigatran. Excretion via the kidneys also decreases with increasing
age.2,4
Creatinine clearance should be checked in all patients before treatment with dabigatran (see Page 15). Patients with
severe renal impairment (creatinine clearance < 30 mL/min) should not be prescribed dabigatran.2 Patients with
this level of renal impairment were excluded from clinical trials and dabigatran datasheets list this as a contraindication.2,3,4
Any patient taking dabigatran, who has renal impairment or is at risk of developing renal impairment, should have their
eGFR checked or creatinine clearance calculated every six to 12 months during long-term treatment.6 In some
patients, more frequent checks may be appropriate. If a patient develops acute renal failure while taking dabigatran it
should be stopped.2
The remaining 20% of the medicine is eliminated via the liver.4 Although hepatotoxicity has not been demonstrated
with dabigatran, caution is advised when it is used in patients with severe liver disease. Patients with active liver
disease or persistently raised liver enzymes (> two times upper limit of normal) were excluded from clinical trials.2,3 Earlier
types of direct thrombin inhibitors failed to reach clinical use due to hepatotoxicity, e.g. ximelagatran.8
Dabigatran dosing in renal impairment for patients with atrial fibrillation
Creatinine clearance < 30 mL/min – there is no clinical experience of the use of dabigatran in this
group of patients. Dabigatran is currently contraindicated in the New Zealand medicine data sheet, for this group of
patients.
Creatinine clearance 30 – 50 mL/min – use dabigatran with caution in this group of patients.
For patients with non-valvular AF, with creatinine clearance 30 – 50 mL/min, there are no specific recommendations
to reduce the dose of dabigatran from 150 mg, twice daily. However, patients with renal function in this range may be
at increased bleeding risk due to reduced dabigatran excretion, especially if other risk factors are present. Some practitioners
recommend using a lower dose of 110 mg dabigatran, twice daily. However, it is not known if this dose is safer and evidence
shows that it is likely to be less effective than the 150 mg dose.
The decision whether to prescribe dabigatran for patients in this group, and at what dose, should be individualised,
with consideration given to factors such as the patient’s overall bleeding risk and their specific creatinine clearance
level. Discussion with a cardiologist may be helpful. Recommendations are likely to become clearer as more clinical experience
becomes available with this medicine.
Twice daily dosing is required
Dabigatran has a short half life of approximately 12–14 hours in adults with normal renal function.2 In people
with impaired renal function, the half life is prolonged.2 Regular twice daily dosing with an interval of approximately
twelve hours is required. Efficacy is likely to be compromised with poor adherence.6 Patients should be made
aware that good compliance is important to sustain clinically effective anticoagulation.
There is limited evidence on the clinical effect of a missed dose. It is advised that:2,4
- If a dose is missed, the dose can be taken when the patient remembers, provided it is more than six hours until the
next scheduled dose
- If it is within six hours of the next scheduled dose, the patient should be advised not to take the missed dose
- A double dose should not be taken to make up for a missed dose
Dabigatran is not affected when taken with food
Although there is evidence that meals high in fat may delay the time taken to reach peak concentration in the plasma
by approximately two hours, this does not appear to affect the bioavailability and clinical effectiveness of dabigatran.2,8 The
capsules can therefore be taken with water, with or without food. Advising patients to take the capsules with breakfast
and the evening meal may help with compliance.
The capsules should be swallowed whole and not chewed, or opened to sprinkle the contents on food or in fluids, as this
significantly increases (75%) the oral bioavailability and may therefore increase the risk of bleeding.2
How do warfarin and dabigatran affect coagulation?
All anticoagulant agents work by inhibiting the activity of thrombin. Thrombin enables the conversion of fibrinogen
into fibrin during the coagulation cascade, therefore its inhibition prevents the development of thrombus.
The anticoagulatory effect of warfarin is due to inhibition of several components of the coagulation pathway including
vitamin K-dependent factors II, VII, IX and X, and proteins C and S, therefore indirectly inhibiting thrombin. Dabigatran,
in contrast, selectively and directly inhibits thrombin (Figure 1).7
By inhibiting thrombin, dabigatran prevents a number of processes in the coagulation pathway including:6
- The conversion of fibrinogen into fibrin
- Positive feedback amplification of coagulation activation
- Cross-linking of fibrin monomers
- Thrombin-induced platelet activation
- The inhibition of fibrinolysis
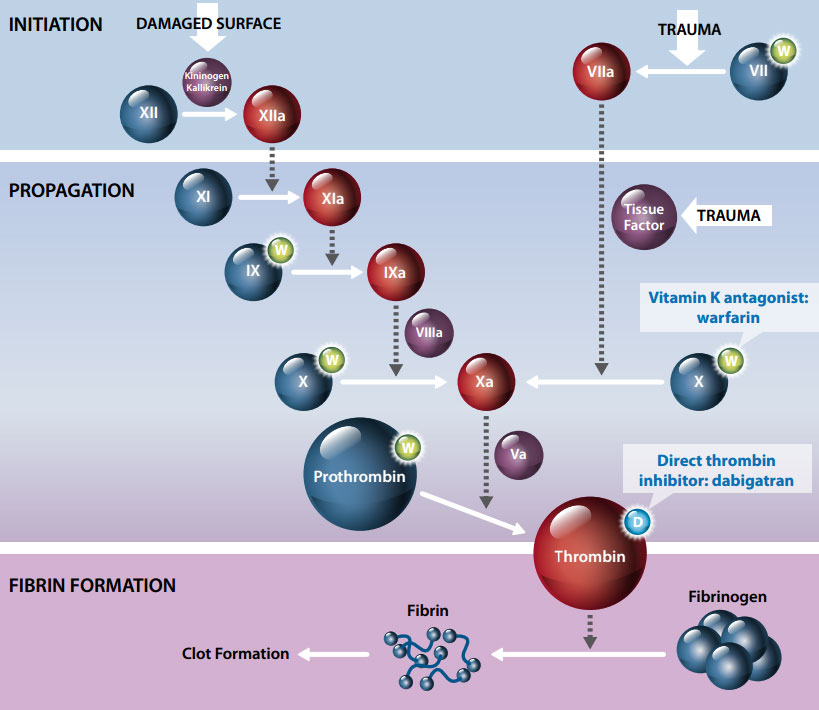
Figure 1. Coagulation cascade showing site of action of anticoagulants warfarin and
dabigatran
Dabigatran cannot be re-packaged into blister packs
Dabigatran capsules must be used within 30 days once the bottle is opened. If exposed to moisture the capsules have
the potential to break down and there is a risk of loss of potency.2,10 It is recommended that the capsules
are stored in their original bottle, with the lid tightly closed, to protect from moisture. The lid of the bottle contains
a desiccant to help prevent moisture affecting the capsules. The manufacturer has recommended to pharmacies that dabigatran
should not be re-packaged into weekly blister packs. New packaging to overcome this issue is likely to be supplied in
the future.
What are the interactions with other medicines?
The knowledge on medicine and dietary interactions involving dabigatran is still in its infancy and few clinically significant
interactions have been reported.2,11 Table 1 lists the major medicine interactions that are currently known.
Unexpected or even potentially life-threatening medicine interactions may be identified with more widespread and prolonged
use.5 Vigilance is therefore required when initiating dabigatran or when any changes in the patient’s
medicine profile are made.
Metabolism of dabigatran etexilate to its active form does not use cytochrome P-450 pathways, which reduces the likelihood
of drug-drug and drug-diet interactions.2,6 Dabigatran etexilate (the prodrug) is a substrate for the efflux
transporter P-glycoprotein (P-gp) although the active medicine dabigatran is not.2 Therefore there is the potential
for interactions with medicines that are substrates, inhibitors or inducers of P-gp (Table 1, over page).2,8,11
Dabigatran is contraindicated in patients taking oral ketoconazole, a P-gp inhibitor.2 Although no dose adjustment
is recommended in the New Zealand datasheet, dabigatran should be used with caution in patients taking amiodarone or verapamil
(also P-gp inhibitors).2,9 Some experts advise that patients take dabigatran two hours before taking verapamil
and antacids.4,9 However, this may be impractical and using an alternative medicine may be a safer course of
action until there has been more clinical experience with dabigatran.
Table 1. Summary of known dabigatran interactions2,4,9,11
| Interaction |
Medicine |
Clinical considerations |
Agents that increase gastric pH, decrease absorption:
 dabigatran concentration dabigatran concentration |
Antacids |
No clinically significant reduction in plasma concentration has been shown with concomitant
use of antacids. Two hour separation of dabigatran and antacids is advised by some, or use an alternative medicine. |
| Proton-pump
inhibitors* |
Pantoprazole has been shown to reduce the plasma concentration of dabigatran by up to 30% and
similar effects would be expected with other PPIs such as omeprazole. A subgroup analysis of the RE-LY trial indicated
that the interaction is not clinically significant and that the combination of a PPI and dabigatran need not be avoided.
Further studies are required. |
P-gp inhibitors:
 dabigatran concentration dabigatran concentration |
Amiodarone
Verapamil |
Amiodarone and verapamil have been shown to increase the plasma concentration of dabigatran
and although no dose adjustment is generally recommended, this combination of medicines should be used with caution.
Two hour separation of dabigatran is advised by some but switching to an alternative medicine may be preferable,
particularly for patients on verapamil |
| Digoxin |
Concomitant use of digoxin with dabigatran has been shown to result in a small, non-clinically
significant, increase in plasma concentration. However, in practice this combination appears safe and well tolerated |
CYP3A4 and P-gp inhibitors:
 dabigatran concentration dabigatran concentration |
Ketoconazole |
Concurrent use of dabigatran with oral ketoconazole is contraindicated due to a marked increase
in plasma concentration |
| Clarithromycin |
No dose adjustment is recommended for clarithromycin although it is known to cause a non-clinically
significant increase in plasma concentration |
CYP3A4 and P-gp inducers:
 dabigatran concentration dabigatran concentration |
Rifampicin |
Avoid concurrent use of dabigatran with rifampicin if possible as this strong P-gp inducer significantly
reduces the plasma concentration of dabigatran |
| Carbamazepine |
This P-gp inducer is expected to also reduce the plasma concentration of dabigatran and should
be avoided or used with caution |
Antiplatelet agents:
 anticoagulant effect anticoagulant effect |
Aspirin
Clopidogrel |
No dose adjustment is recommended, however, a cautious approach is necessary. Clopidogrel has
been shown to increase plasma concentration and in the RE-LY trial the use of antiplatelet agents doubled the risk
of major bleeding (although this also applied to warfarin). Current expert opinion is that these medicines should not
be used with dabigatran, although in secondary care their use may be considered on a case by case basis. |
NSAIDs:
 bleeding risk bleeding risk
 antiplatelet effect antiplatelet effect |
All
NSAIDs** |
No dose adjustment is recommended
Concurrent administration of NSAIDs may increase the risk or severity of a bleed. Monitor for any abnormal bleeding |
St John’s wort:
 dabigatran concentration dabigatran concentration |
St. John’s wort preparations |
This P-gp inducer is expected to reduce the plasma concentration of dabigatran.
Avoid or use with caution. |
P-gp = P-glycoprotein, CYP3A4 = cytochrome P450 3A4, NSAIDs = non-steroidal anti-inflammatory drugs
Key clinically relevant features from Table 1:
- Antiplatelet agents and NSAIDs (both conventional and Cox-2) should be used with caution in people taking dabigatran
because the risk of bleeding may be increased.2 Evidence shows that people taking dabigatran concomitantly
with aspirin or clopidogrel have approximately double the risk of major bleeding, irrespective of the dose.1,2 (N.B.
a similar risk applies to patients taking warfarin). Patients taking these medicines or NSAIDs should be monitored clinically
for signs of bleeding, e.g. ask about bleeding noses, wounds that keep bleeding, gums that are bleeding more than usual.
Some patients may require an intermittent check for anaemia.
- The use of dabigatran with oral ketoconazole is contraindicated because clinical trials have shown that ketoconazole
increases the maximum plasma concentration by approximately 150%.2
- Amiodarone and verapamil are medicines that are used in a similar population of people to those that require anticoagulation.
A cautious approach should be taken as there is evidence that if amiodarone and verapamil are taken within two hours
of dabigatran, the plasma concentration of dabigatran increases.2,9 Clinical use over time may help determine
whether this increase produces clinically significant adverse effects with combinations of these medicines.
- Proton pump inhibitors do not appear to affect the anticoagulant efficacy of dabigatran.2,12
There are no known food interactions with dabigatran and there has been no direct interaction between alcohol and dabigatran
in animal models.4
Calculating creatinine clearance
Most laboratories report eGFR automatically with serum creatinine results, and eGFR can be used as an estimate of renal
function. However, eGFR may not be a good estimate of renal function in people at extremes of body size (BMI < 18.5 or > 30
kg/m2) or in older people. In this case, an estimate of creatinine clearance is preferable, determined using
a hand held or electronic calculating tool or by using the Cockcroft-Gault equation:
| Creatinine clearance (mL/min) = |
(140 – age) x weight (kg) x constant
serum creatinine (µmol/L). |
The constant = 1.23 for men, 1.04 for women.
Is there any need for routine coagulation monitoring?
Routine coagulation monitoring is not required for patients taking dabigatran because of the rapid onset of action,
a wide therapeutic window and predictable pharmacokinetics and pharmacodynamics.13,14 There is currently no
test available to routinely guide dabigatran dosage. In particular, dabigatran has variable and unpredictable effects
on INR, which is not useful for monitoring.14
If a patient taking dabigatran experiences bleeding symptoms, the following should be considered:
- Is the patient taking any other medicines that affect coagulation, e.g. aspirin?
- Is the patient taking any medicines known to interact with dabigatran?
- Does the patient have impaired renal function, or has renal function deteriorated?
Management of bleeding complications in patients taking dabigatran should be individualised according to the site and
severity. Dabigatran should be stopped and the source of bleeding investigated. Unless the bleeding is mild and able to
be managed within the community, patients with bleeding should be referred urgently to secondary care (see Figure
2).
If bleeding is a problem for a patient on dabigatran, what laboratory tests can be used to assess coagulation?
The activated partial thromboplastin time (aPTT) and thrombin time (TT) can be used to guide management of patients
with acute bleeding, but these tests are not suitable for fine tuning dabigatran dosage.13,14 These tests can
indicate whether dabigatran is “on board”, i.e. whether there is anticoagulant activity, e.g. if compliance
is an issue or to determine if the medicine has been excreted. The time of the last dose of dabigatran should be included
on the blood request form as this is critical for interpreting results.
Activated Partial Thromboplastin Time (aPTT) – this test does not have a linear relationship with drug
levels. The test is moderately sensitive to the effect of dabigatran but the response is blunted at higher doses.
Thrombin Time (TT) – at recommended doses, dabigatran increases TT. This test is very sensitive and
although there is a linear dose-response relationship, the time is very prolonged at therapeutic doses and the effect
is also method specific making results potentially difficult to interpret.
The Ecarin clotting time (ECT) – this test is sensitive and has a linear dose-response relationship
but is not widely available in New Zealand.
The primary role of these tests is to give a general guide as to whether a patient taking dabigatran, who is bleeding,
still has a significant anticoagulant effect from the medicine. If neither the aPTT nor TT is prolonged there is no significant
residual anticoagulant activity.14 If the TT only is prolonged, there is some residual anticoagulant effect,
but at a low level only. If both tests are prolonged there is likely to be a significant effect from dabigatran present
(or another haemostatic defect).13,14
Other tests to monitor coagulation status in patients taking dabigatran are being developed, however, they are not widely
available and require standardisation for use.
Dabigatran may also have an effect on a number of other coagulation tests and its use should be recorded on the request
form if a patient taking dabigatran requires any coagulation test such as thrombophilia markers and lupus anticoagulant
testing.
Adverse effects of dabigatran – bleeding is the most relevant
All anticoagulant medicines inherently increase the risk of bleeding and patients should be informed of the risks and
advised to let their General Practitioner know if they have any concerns.
The most common adverse effect with dabigatran is bleeding and the risk of major bleeding is comparable to that of warfarin.1 In
the RE-LY trial, dabigatran (150 mg or 110 mg), caused fewer intracranial haemorrhages and life-threatening bleeds when
compared to warfarin, however, rates of major gastrointestinal bleeding were higher for patients on dabigatran than those
on warfarin.1 Overall the bleeding risk for patients taking dabigatran is greater at the higher dose of 150
mg, twice daily, and decreases when lower doses are used.
 For more information regarding the risk of intracranial haemorrhage in dabigatran, see "Correspondence: An international perspective on the use of dabigatran", BPJ 52 (April, 2013).
For more information regarding the risk of intracranial haemorrhage in dabigatran, see "Correspondence: An international perspective on the use of dabigatran", BPJ 52 (April, 2013).
Major or severe bleeding, regardless of location, may lead to disabling, life-threatening or even fatal outcomes. Dabigatran
should not be used in patients with clinically significant bleeding or who are at high risk for bleeding.
There is no antidote for bleeding from dabigatran, unlike vitamin K for warfarin. If haemorrhagic complications occur
treatment should be stopped.
 For advice about tools to estimate stroke and bleeding risk, see: “The
warfarin dilemma”, BPJ 31 (Oct, 2010).
For advice about tools to estimate stroke and bleeding risk, see: “The
warfarin dilemma”, BPJ 31 (Oct, 2010).
Dyspepsia is a commonly reported adverse effect with dabigatran. In the RE-LY trial, 11.8% of people
taking 110 mg, twice daily, and 11.3% of patients taking 150 mg, twice daily, experienced dyspepsia compared with 5.8%
in patients taking warfarin.1 Each capsule contains a tartaric acid core, because absorption of dabigatran
elexilate requires an acid environment.6,11 It is thought that the acid core may contribute to the development
of dyspepsia. Dabigatran, therefore, may not be well tolerated particularly in patients with a history of gastrointestinal
problems.5,11
Rates of myocardial infarction may be higher
The incidence of myocardial infarction (MI) in the RE-LY trial was significantly lower in patients in the warfarin group
compared to the dabigatran group.1 Some evidence suggests that dabigatran may not actually increase the risk
of MI but rather that warfarin provides a protective effect.2,15 Whether dabigatran poses a genuinely increased
risk of MI is still unclear.5
What adjustments in dabigatran dose are required for operative procedures?
At present there is limited evidence and clinical experience with the use of dabigatran prior to surgery. It is anticipated
that the risk of bleeding with dabigatran is likely to be similar to the risk for a patient taking warfarin. However,
it should be considered that prolonged bleeding times with dabigatran cannot be reversed, unlike warfarin (with vitamin
K able to be used).
Planning has always been required for patients taking warfarin and the situation will be no different for patients taking
dabigatran. Good communication should be maintained between primary and secondary care so clear consistent instructions
for patients can be given and followed. The bleeding risk, the type of surgery planned and the renal function of the patient
should be considered.
For people with a standard risk of bleeding, dabigatran should be temporarily discontinued for 24 to 48 hours before
elective surgical procedures.2,6 For people at increased risk (e.g. older people, concomitant use of antiplatelet
medicines, cardiac, respiratory or liver disease) or those having procedures with a high bleeding risk (e.g. any major
surgery, spinal anaesthesia), dabigatran should be discontinued two to four days prior to the surgery.2 If
the risk of bleeding is high, a normal aPTT result will indicate a lack of residual anticoagulant effect.13,14
Warfarin does not need to be stopped for some procedures such as dental extractions and minor surgery if the patient’s
INR value is at the lower end of the therapeutic range and their individual risk of bleeding is low. There is limited
information about the use of dabigatran in this situation, but it can be assumed that a similar assessment of risk can
take place, although bearing in mind that a bleeding event with dabigatran cannot be reversed.
Evidence shows that dabigatran can be used safely in patients undergoing cardioversion.2,16
Reporting patient bleeds with dabigatran
 The Haematological Society, in association with Medsafe, PHARMAC and
the Centre for Adverse Reactions Monitoring (CARM) is collecting data about adverse bleeding events experienced by patients
using dabigatran.
The Haematological Society, in association with Medsafe, PHARMAC and
the Centre for Adverse Reactions Monitoring (CARM) is collecting data about adverse bleeding events experienced by patients
using dabigatran.
Dr Paul Harper, consultant haematologist at Palmerston North Hospital is co-ordinating this review. He asks that all
patient bleeds, adverse events or discontinuation of therapy with dabigatran (Pradaxa) be reported to CARM. Events should
be reported regardless of whether the patient required hospitalisation. If in doubt, report – it is not necessary to
be certain that an adverse reaction is caused by a medicine in order to make reporting worthwhile.
Adverse reaction reports should include as much information as possible, and can be made via:
- bestpractice Decision Support – click “Adverse drug reaction reporting” under the module list
- Or reporting cards – found inside the back cover of Prescriber Update and with the MIMS Catalogue
- Or directly with CARM, online at: https://nzphvc-01.otago.ac.nz/carm phone:
03 479-7247, fax: 03 479-7150 or email: carmnz@otago.ac.nz
 The Safe and Quality Use of Medicines Group (SQM) has published an urgent
alert following hospital admissions for the treatment of bleeding after dabigatran initiation. This report can be found
on the SQM website at: www.safeuseofmedicines.co.nz
The Safe and Quality Use of Medicines Group (SQM) has published an urgent
alert following hospital admissions for the treatment of bleeding after dabigatran initiation. This report can be found
on the SQM website at: www.safeuseofmedicines.co.nz
How can bleeding be managed for people taking dabigatran?
Unlike warfarin and heparin, no specific antidote is available to reverse the anticoagulant effects of dabigatran. Administration
of vitamin K or an infusion of plasma will not reverse the anticoagulant effect.
Unless the bleeding is mild, it is anticipated that most patients will require referral to secondary care for urgent
treatment, although this will depend on individual patients and the location and severity of the haemorrhage. Treatment
in secondary care may involve the use of oral charcoal (if ingestion of dabigatran was less than two hours previous),
transfusion of blood products or clotting factors, use of anti-fibrinolytic agents intravenously and consideration of
haemodialysis, particularly if there is moderate to severe renal impairment (Figure 2).14
Severe or life-threatening bleeding may be immediately obvious due to the clinical state of the patient, e.g. tachycardia,
pallor, hypotension, bleeding with injury. However, some patients, particularly younger patients, may have normal vital
signs, even with a significant blood loss. In addition, there may be bleeding within a body cavity, e.g. stomach, bowel
or chest, that is not clinically detectable until a large volume of blood has been lost. Although an urgent complete blood
count to assess the haemoglobin level may be useful, in a general practice setting, unless the bleeding is mild, referral
to secondary care is recommended for patients taking dabigatran who are bleeding. As a guide, the categories used in the
RE-LY trial to define the severity of bleeding were; a decrease of 20g/L Hb signifying moderate to severe bleeding and
a decrease of 50g/L Hb, life-threatening bleeding.1
 Details of the management of moderate, severe or life-threatening bleeding
is available from: www.pharmac.govt.nz/2011/06/13/Dabigatran
bleeding management.pdf.
Details of the management of moderate, severe or life-threatening bleeding
is available from: www.pharmac.govt.nz/2011/06/13/Dabigatran
bleeding management.pdf.
Dabigatran associated bleeding
| Identify the site and cause of bleeding if possible |
 |
| Assess the severity of the bleeding using clinical
signs and CBC if indicated |
 |
Check aPPT and TT, fibrinogen assay, creatinine and
electrolytes, calcium
– Note the time of the last dabigatran dose on the request form
Consult with a haematologist for advice about ongoing management |
 |
Stop dabigatran
– either delay the next dose if bleeding is mild or discontinue if bleeding is more severe |
| |
| Mild bleeding |
For moderate, severe or life-threatening bleeding* |
If applicable elevate affected body part and apply compression
Consider use of oral tranexamic acid (15 mg/kg, four times per day)
Ensure good fluid intake to maximise renal excretion |
Refer urgently to hospital
Measures as for mild bleeding
Initiate standard resuscitation measures if required (e.g. establish IV access, give IV fluids, oxygen) |
| * |
Moderate to severe bleeding – a reduction in Hb≥20g/L, symptomatic bleeding in an organ or critical
area, e.g. intraocular, intracranial, intramuscular, retroperitoneal, intraarticular or pericardial bleeding.2 |
| |
Life-threatening bleeding – a reduction in Hb≥50g/L, symptomatic intracranial bleed, hypotension
requiring inotropic agents, e.g. dopamine, bleeding requiring surgery2 |
|
Figure 2: Treatment of dabigatran associated bleeding in primary care (adapted from
van Ryn14)
Initiating dabigatran or switching between oral anticoagulants
Initiation in patients not previously anticoagulated with warfarin
No loading dose is required when initiating dabigatran, the medicine is started and continued at the same dose.2
How do you change from warfarin to dabigatran?
Stop warfarin and start dabigatran when the INR is less than 2.0.2
How do you change a patient from dabigatran to warfarin?
Check the creatinine clearance. Warfarin should be started three days prior to stopping dabigatran if the creatinine
clearance is > 50 mL/min. If the creatinine clearance is 30 – 50 mL/min, start warfarin two days before stopping dabigatran.2
 Best Practice tip: If switching a patient from warfarin to dabigatran,
notify the local laboratory by phone or email so that they can update their records and avoid unnecessary INR testing.
Patients taking warfarin are often registered with a laboratory for regular, long-term repeat INR’s.
Best Practice tip: If switching a patient from warfarin to dabigatran,
notify the local laboratory by phone or email so that they can update their records and avoid unnecessary INR testing.
Patients taking warfarin are often registered with a laboratory for regular, long-term repeat INR’s.
The evidence for dabigatran – can we RE-LY on this?
The Randomised Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial was a “non-inferiority” trial.1 In
this type of trial, a new medicine is compared with a current standard treatment in an attempt to determine whether the
new medicine is no worse than the usual medicine.18 The new medicine does not have to be superior to the older
medicine. In contrast, randomised trials usually assess if a new medicine is better than a current medicine or placebo
and are called superiority trials.
The RE-LY trial therefore had to show that outcomes for the people who took dabigatran were at least as good as the
outcomes for the people who took warfarin.
Summary of findings from the RE-LY trial
This large, randomised, non-inferiority clinical trial compared two doses of dabigatran (110 mg and 150 mg administered
twice daily) to warfarin treatment (aiming for INR values of 2–3) in over 18,000 patients with atrial fibrillation.1 The
study was of hybrid design with medicine administration blinded for patients on dabigatran but not for warfarin.
Compared to warfarin, the 150 mg, twice daily dose of dabigatran significantly reduced the rate of stroke or systemic
embolism.1 The 150 mg dose was therefore found to be superior to warfarin for the prevention of stroke or
systemic embolism. There was no significant difference in the rate of stroke or systemic embolism with the 110 mg, twice
daily dose of dabigatran when compared to warfarin. Twice daily dabigatran 110 mg was therefore found to be non-inferior
to warfarin.1
Both doses of dabigatran were associated with fewer intracranial haemorrhages and other life-threatening bleeds when
compared to warfarin, however, gastrointestinal bleeding events were significantly increased with the higher dose of
dabigatran.1 The rate of myocardial infarction was significantly higher (p = 0.048) in patients in the dabigatran
group.1
There were no significant differences in the mortality rates from any cause between either of the dabigatran treatment
groups and the warfarin group.1
| Event |
% of incidents per year
|
Significance (P ≥ 0.05) |
| |
Dabigatran 110 mg |
Dabigatran 150 mg |
Warfarin |
|
Stroke or systemic embolism
|
1.53 |
1.11 |
1.69 |
D150 superior to W
D110 not inferior to W
D150 superior to D110 |
| Myocardial infarction |
0.72 |
0.74 |
0.53 |
W superior to D150 |
| Intracranial haemorrhage |
0.23 |
0.30 |
0.74 |
D110 superior to W
D150 superior to W
|
| Life-threatening bleeding |
1.22 |
1.45 |
1.80 |
D110 superior to W
D150 superior to W
|
| Gastrointestinal bleeding |
1.12 |
1.51 |
1.02 |
W superior to D150
D110 superior to D150 |
| Death from vascular causes |
2.43 |
2.28 |
2.69 |
D150 superior to W |
| Death from any causes |
3.75 |
3.64 |
4.13 |
No difference |
What were the strengths of the RE-LY trial?
The trial was large, including over 18,000 patients from multiple countries. Follow up of participants was excellent
with 99.9% of patients completing follow up assessments over a median time frame of two years.1 Patients were
allocated randomly into the three treatment groups (dabigatran 110 mg twice daily, dabigatran 150 mg twice daily or warfarin).
Administration of dabigatran was blinded, however, warfarin was not because of the need for INR monitoring. The investigators
were aware of this potential for bias and therefore implemented strategies to minimise bias such as arranging for assessment
of the outcomes to be carried out by two independent parties who had no knowledge of the treatments received.
What were the limitations of the RE-LY trial?
This was an industry funded trial, however, the coordination of the study, data management and analysis of the results
were carried out on an independent basis at McMaster University in Canada.1,19
The study participants represented a select group of people and the outcomes of treatment with dabigatran may be different
in a “real world” setting.20 Participants had AF and a minimum of one other risk factor for stroke,
e.g. previous stroke, hypertension, coronary artery disease.3 People excluded from the study included those
with:3
- Haemodynamically significant valvular heart disease or a prosthetic valve
- Any stroke in the previous two weeks or a severe disabling stroke in the last six months
- An increased risk of bleeding, e.g. GI bleeding within the previous year, documented GI ulcer within the last month,
major surgery within the last month, uncontrolled hypertension, a history of bleeding, any haemorrhagic disorder
- Severe renal impairment (creatinine clearance ≤ 30 mL/min)
- Active liver disease
- Anaemia or thrombocytopaenia
Although the administration of dabigatran was blinded, participants receiving warfarin could not be administered this
medicine in a blinded manner due to the need for INR measurement and subsequent adjustment of doses. There have been
comments in the literature stating that this may have altered the way patients in the warfarin arm of the trial were
managed, i.e. performance bias.21,22
The standard of anticoagulation in patients on warfarin, with INR values in the therapeutic range for 64% of the time,
has been said to be poorer than that achieved in many centres, although the level is similar to that achieved in most
randomised controlled trials.22,23 In addition, the INR values at which adverse events occurred were not reported.
Some researchers believe that the benefits reported for dabigatran would be minimised if they were compared with patients
taking warfarin who had INR values consistently within the therapeutic range.22 To address some of the questions
regarding INR control raised by the United States Food and Drug Administration and other researchers, a subsequent analysis
of RE-LY data has reported that the primary outcomes remained consistent irrespective of the quality of INR control.21,24
Other concerns that have been raised include the higher rates of withdrawal due to adverse effects in the dabigatran
arms of the study and the concomitant use of antiplatelet agents in all three arms of the trial.21
At this stage the longer term effects (post two years) of dabigatran are not known although there is an ongoing multi-centre
follow up study in place (RELY-ABLE).
There is still a lot to learn about dabigatran
Dabigatran may well provide a solution to some of the problems associated with the use of warfarin such as its unpredictable
and significant inter-individual variability in response and narrow therapeutic window which necessitates frequent INR
monitoring as well as numerous food and medicine interactions.5 However, the importance of the frequent patient
contact that accompanies INR monitoring should not be forgotten as this often goes beyond “a simple blood test”.
The consequences of long-term use of dabigatran are unknown and this may be important in the setting of stroke prevention
in patients with atrial fibrillation as these patients usually require life-long treatment.5,6 Thrombin plays
an important role not only in coagulation but also in immune response, infection, angiogenesis, endothelial function,
and tumour growth.6
The main clinical trial (RE-LY), which has prompted the review of recommendations in atrial fibrillation guidelines,
included just over 18,000 people who took dabigatran for two years.1 There is still a lot to learn about dabigatran
– its effectiveness, adverse effects, longer term safety and interactions with other medicines. This information will
only be gathered once it has been used extensively over the next few years.
Like all medicines the promise that dabigatran brings must be balanced against its potential risks and uncertainty,
therefore a cautious approach to its use is recommended.