For further information on the role of opioids in chronic pain see:
Understanding the role of opioids in chronic non-malignant pain bpacnz, October 2018
In this article 
View / Download
pdf version of this article
| Key reviewers: |
Dr Leanne Parker, Empower Rehabilitation / ACC
Dr Rick Acland, Director of Medical Services, Burwood Hospital
Dr Tom Swire, Palliative Care Specialist, Dunedin |
| Key concepts |
- Ask about pain
- Diagnose the type of pain (nociceptive or neuropathic) and the source of pain
- Use individualised pain scales to assess the severity of pain and its affect on function
- Manage pain using the WHO analgesic ladder – moving from simple non-opioid analgesia up to potent opioids
- Remember “ABC” when prescribing opioids – consider prescribing an antiemetic for nausea, prescribe breakthrough
pain doses and prescribe laxatives for constipation
|
Understanding pain
Pain is a common problem. Patients tell us if they have acute pain they are often vocal, upset and asking for help.
In contrast, chronic pain is often hidden and unless we ask patients we may not find out they are in pain. The burden
of chronic pain is in its impact on daily life.
“Think of pain as the fifth vital sign”
Managing pain includes helping the patient understand why they have pain, creating realistic expectations for relief
and treating the pain.
Although not covered in this article, the psychological aspects of care are paramount. Factors such as anxiety and depression,
which may reduce tolerance to pain or be exacerbated by pain, must also be assessed and treated.
There are two types of pain
From a practical point of view, pain is either generated by damage to the nerves (neuropathic pain), or by damage
to other tissues (nociceptive pain). The anatomical source of the pain should be identified and this will then guide
treatment. It is important to be aware that people can have more than one source or type of pain at one time.
The relationship between a painful stimulus and the experience of pain is extremely complex. Modification of the stimulus
occurs in the peripheral and central nervous systems. The final perception of pain is strongly influenced by emotion and
cognition.
Recognition of pain
Ask.
Diagnosis of pain
Ask more.
- How bad is the pain?
- Where is it? Does it go anywhere?
- What makes the pain worse? What makes it better?
- How is the pain described? e.g. dull, sharp
- How does it impact on daily life?
Signs of pain in people with communication difficulties1
For people with communication difficulties (e.g. dementia, confusion, coma, learning
difficulty) diagnosing pain may not be as simple as asking them. Pain can cause a variety of behaviours and signs.
Change in behaviour is a key indicator.
Examples of pain behaviours and signs:
- Expressive: grimacing, clenched teeth, frowning, eyes open wide or shut, crying, sighing, moaning.
|
- Adaptive: rubbing or holding area, keeping area still.
- Distractive: rocking, pacing, biting, clenching fists.
- Postural: flinching, head in hands, limping.
There are separate pain scales for children and for patients in intensive care and long term care facilities. |
Pain scales can help to determine severity
Pain is what the patient says it is. It cannot be measured directly but pain scales may be used to assess severity.
The most clinically useful pain scales include an assessment of impact on daily life.
An example of this is the Support Team Assessment Schedule (STAS) pain module2
Measurement of effect of pain on patient:
- 4 = Severe and continuous overwhelming pain. Unable to think of other matters.
- 3 = Severe pain often present. Activities and concentration markedly affected by pain.
- 2 = Moderate distress, occasional bad days, pain limits some activities.
- 1 = Occasional grumbling, single pain. Patient not bothered to be rid of symptom.
- 0 = None.
Scales can be made even more useful if they are personalised. For example, in the case of a patient with severe arthritis
in their hip, level four could be continuous pain day and night with the patient being unable to sleep, level three could
be the patient is able to sleep but is still troubled with pain while they are awake and so on. These scales can be used
to set individual goals.
Other commonly used pain assessment tools include:3
- Numerical rating scale: pain is rated on a scale from 0 (no pain) to 10 (worst pain imaginable).
- Verbal rating scale: a four-point scale in which pain is rated as none, mild, moderate or severe.
- Visual analogue scale: an unmarked line with “no pain” at one end and “worst pain imaginable” at
the other end.
- Faces pain scale: There are several versions available showing smiling or neutral faces for no pain and sad or crying
faces indicative of severe pain.
So what is causing the pain?
In order to manage pain, work out which tissue the pain is coming from. At its simplest, this divides neuropathic pain
from nociceptive pain. Nociceptive pain then needs to be diagnosed further using the same process as with any differential
diagnosis.
For example, chest pain could be:
- Sharp and burning with associated allodynia (sensation of pain due to light touch). Diagnosis: neuropathic pain, query
shingles.
- Pain worse on movement, tender to touch, associated with bruising. Diagnosis: nociceptive pain, soft tissue injury.
- Sharp pain worse on breathing, associated crackles on auscultation and fever. Diagnosis: nociceptive pain, pleurisy.
- Central, crushing pain, worse on exercise, associated dyspnoea. Diagnosis: nociceptive pain, ischaemia.
- Central, burning pain, worse after eating. Diagnosis: nociceptive pain, oesophageal irritation.
- Tight, hot, burning sensation localised around wound. Diagnosis: nociceptive pain, skin infection.
Pharmacological treatment of pain
Medications that may be considered for treating pain include drugs that treat specific conditions (adjuvants) and drugs
in the analgesic ladder. There is one ladder for nociceptive pain (see below) and one for neuropathic
pain.
Pain control is not always easy. It can be complicated by psychological and addiction problems. Adverse effects of medications
may be problematic. Don’t hesitate to seek advice from a specialist.
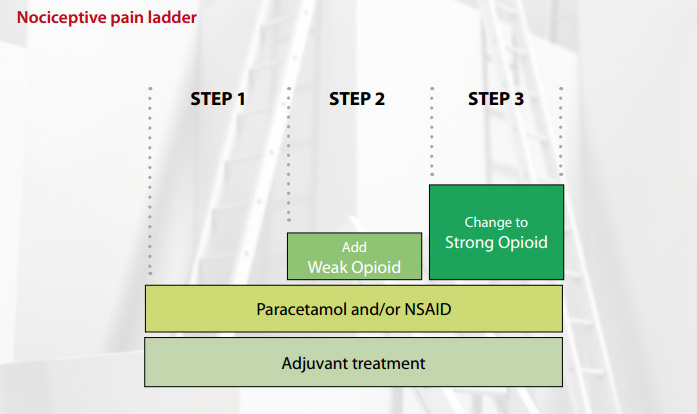
The WHO analgesic ladder for nociceptive pain
The WHO analgesic ladder4 follows a simple step approach to pain management, starting with a non-opioid and
moving up to potent opioids. It is important to be guided by the following rules:
- By the mouth – oral medication
- By the clock – regular medication
- By the ladder – use the analgesic ladder
- Individual dose titration – find the right dose for the patient
- Use adjuvant drugs – treat specific conditions
- Attention to detail – keep reviewing the diagnosis and check for adverse effects
Adjuvants are used at every step of the ladder
Adjuvants are included at every level of the analgesic ladder and may be the only analgesia needed for some conditions.
Some examples of adjuvants:
- Radiotherapy, bisphosphonates – pain from bone metastases
- Antimuscarinics (hyoscine) – bowel colic
- Antibiotics – cellulitis
- Glyceryl trinitrate spray – ischaemia
- Steroids – liver capsular pain
| ABC for prescribing opioids |
A – consider an antiemetic. Opioids may produce nausea at the beginning of treatment.
In some cases, it may be ongoing. Opioid related nausea is produced through two effects - the first is reduction in
gut motility which can be successfully treated with metoclopramide 10mg, three to four times per day. The second is
a central effect which is traditionally treated with haloperidol 1–2mg, once per day. Doses should be modified for
individual patients.
B – Breakthrough. Analgesics work best with regular dosing, but pain may still break through. When
a regular dose of a strong opioid is prescribed, a short-acting formulation should also be prescribed at one sixth
of the total 24 hour dose, to cover any breakthrough pain. For example, a 24 hour dose of morphine of 60mg would require
a breakthrough dose of 10mg.
C – Constipation. “The hand that prescribes opioids should always prescribe a laxative”.
Unless being used to control diarrhoea, the patient should be prescribed a stimulant plus softener combination laxative
such as docusate sodium with sennosides (Laxsol). |
Step one: paracetamol and/or NSAIDs
Mild pain of many causes will respond to paracetamol. NSAIDs are also effective in treating mild to moderate pain, particularly
if an inflammatory process is involved. If tolerated, it is recommended that these drugs are continued through the analgesic
ladder.
Step two: add in a “weak” opioid
One advantage of weak opioids is that they do not require a controlled drug script. However they all act like morphine
and have the same range of adverse effects. Consider that giving a patient 60 mg of codeine, four times per day, is the
equivalent of 24mg of morphine in 24 hours (Table 1).
The general rules for weak opioid use are:6
- A weak opioid should be added to, not substituted for a non-opioid.
- There is no advantage of changing between weak opioids. Do not “kangaroo hop” from weak opioid to weak
opioid.
- If a weak opioid is inadequate when given regularly, change to a strong opioid (e.g. morphine).
Table 1: Approximate equivalent morphine doses of weak opioids
5
| |
Typical dose (oral) |
Total 24 hour dose |
Equivalent morphine 24 hour dose |
| Codeine |
60 mg, 4 times/day |
240mg |
24mg |
| Dihydrocodeine |
120 mg, 2 times/day |
240mg |
24mg |
| Tramadol |
50 mg, 4 times/day |
200mg |
40mg |
Step three: change to a “strong” opioid
Morphine is the most familiar strong opioid and therefore is first choice. To move a patient from a weak opioid to morphine,
because their pain is not controlled, first work out what their current equivalent morphine dose is. For example 60 mg
of codeine, four times per day, is the equivalent of 24mg of morphine in 24 hours (Table 1).
Therefore, because the pain was not controlled at this dose, a reasonable starting dose of morphine would be 20mg twice
per day.
The dose of morphine is titrated until pain control is achieved or adverse effects are intolerable (Table 2). There
is no top dose of morphine.
| Common initial |
Common ongoing |
Occasional |
Rare |
Nausea and vomiting
Drowsiness
Unsteadiness
Delirium |
Nausea and vomiting
Constipation |
Dry mouth
Sweating
Pruritis
Hallucinations
Myoclonus |
Respiratory depression
Psychological dependence |
| Fears of prescribers |
Two main fears can inhibit the prescribing of opioids.
The primary concern is regarding addiction. Use of opioids in patients with non-malignant chronic pain is associated
with a low risk of addiction (about one in ten thousand patients). Care however should be taken in patients who have
a high risk for addiction and those who are suffering from central sensitisation pain.
The second concern is the fear of respiratory depression. However pain is a physiological antagonist to the central
depressant effects of opioids. Strong opioids do not cause clinically important respiratory depression in patients
in pain if titrated according to pain.
If the pain is relieved, such as in a patient who has had successful orthopaedic surgery for low back pain, opioids
may be slowly withdrawn to avoid withdrawl symptoms. |
There are two ways of titrating:
Method 1:
Add up all the opioids taken in the previous 24 hours including regular and breakthrough doses. Divide this figure
by two to make a new regular twice daily dose. Remember to recalculate a breakthrough dose, e.g. a patient on 30 mg twice
per day who has taken four 10mg breakthrough doses, has a total 24 hour opioid dose of 100mg. The new regular dose would
then be 50mg twice per day, with a breakthrough dose of 10–15mg — one sixth of the total 24 hour dose. Numbers can be
rounded to make simple regimens with available strengths.
Method 2:
Increase the regular dose by 30 – 50%. For example, if a patient is on a total daily dose of 60mg, an increase
of 30% would take this to 80mg and an increase of 50% would take this to 90mg. Choose whatever dose is easiest in terms
of available strengths, e.g. 40mg, twice per day. Recalculate breakthrough dose.
Opioid rotation
Pethidine is not recommended for chronic pain control. Its short duration of action and high peak increases the risk
of addiction. For the same analgesic effect it has more adverse effects. Be aware that 50mg of pethidine is equivalent
to 12.5mg of morphine.
Ongoing adverse effects may necessitate rotating to another strong opioid. Changing to another opioid requires calculating
the equivalent morphine dose that the patient has been using (Table 3). Figures are approximate because individual patients
metabolise opioids differently.
Patient apprehension about the use of morphine or the presence of impaired renal or liver function may necessitate selecting
an opioid other than morphine.
Table 3: Approximate equivalent morphine doses of strong opioids
| |
Potency Ratio |
Notes |
| Oxycodone (oral) |
1.5 – 2 |
E.g. patient taking oxycodone 5mg four times per day, convert this to oral morphine:
- total daily dose × potency ratio
- = 20mg × 1.5 or 2
- = 30–40mg morphine.
|
| Fentanyl patch |
100 – 150 |
Refer to manufacturers instructions for dose conversion (see
here for an example of this). |
| Methadone (oral) |
5 – 20 |
Dose conversion varies depending on duration of use and dose of opioid. If rotating, specialist advice
is needed. |
 Further reading: Chronic Pain: A Primary Care Condition. Available
from www.arthritisresearchuk.org/
Further reading: Chronic Pain: A Primary Care Condition. Available
from www.arthritisresearchuk.org/