 Want to receive Best Practice Bulletin directly to your inbox?
Sign up here.
Want to receive Best Practice Bulletin directly to your inbox?
Sign up here.
Published: 25 June, 2021
Contents
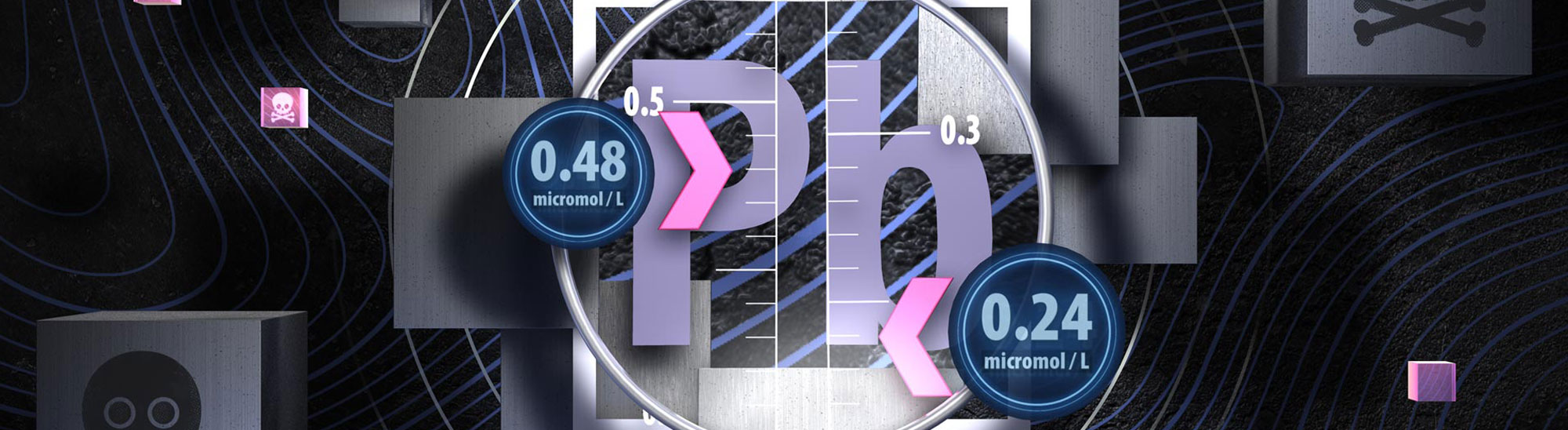
Did you know that blood lead notification levels have now halved?
On 9 April, 2021, the level at which blood lead absorption is required to be notified to the Medical Officer
of Health was reduced to 0.24 micromol/L – half the previous concentration. This change occurred due to mounting evidence
that the level of blood lead that can cause irreversible health effects is lower than previously thought, particularly
in children. Lead notifications can be reported electronically via the Hazardous Substances Disease and Injury Reporting
Tool (HSDIRT) in your patient management system; this helps to contextualise the laboratory result and to direct any
public health responses that are required.
We have just published a new article outlining the notification process and discussing the investigation of a patient with
suspected lead exposure: "Lead absorption notification levels have
reduced".

BPACNZRx prescribing module now available
Is your prescribing module up for renewal? Have you considered BPACNZRx?
This is a new prescribing module available for Medtech 32 and Medtech Evolution. BPACNZRx supports
New Zealand Formulary-based prescribing and incorporates seamless access and integration to the NZ ePrescribing Service. Some features include:
- Access to both adult and child New Zealand Formulary monographs and safety information at the time of prescribing
- A patient-specific medical warning system that considers multiple factors, including current medicine(s), allergies
and other important notes
- Consideration of drug-to-drug interaction(s) utilising Stockley’s Drug Interaction engine; less severe drug-to-drug
interactions can be suppressed at the prescriber’s discretion
- Simple transfer of patient’s existing prescribed medicines into BPACNZRx
Want to find out more or subscribe now? Visit: www.bpac.org.nz/Rx
Omeprazole: new look
From 1 August, 2021, the appearance of Omeprazole Actavis 10 mg and 20 mg capsules and bottles and 40 mg bottles will have changed; the formulation and
manufacturer remains the same. Differences in colour and size between the 10 mg and 20 mg capsules have been implemented to make it easier to distinguish
between doses (Table 1). As omeprazole is one of the most frequently prescribed medicines in New Zealand, this change will affect a large number of patients.
Both prescribers and community pharmacists should ensure that the change is effectively communicated to patients and use the opportunity to reiterate
to patients that if their medicine ever looks different from what they are used to, they should ask about it.
Table 1: Changes in the appearance of Omeprazole Actavis 10 mg and 20 mg capsules
Omeprazole Actavis 10 mg |
Omeprazole Actavis 20 mg |
Current |
New |
Current |
New |
Opaque white |
Opaque yellow |
Opaque white |
Opaque white |
Size 3 |
Size 3 |
Size 2 |
Size 2 |
Printed with “OM” and “10” in black ink |
Not printed |
Printed with “OM” and “20” in black ink |
Not printed |
 |
 |
 |
 |
Varenicline out of stock until late July
PHARMAC has been advised that varenicline will be out of stock from mid-June until the end of July, 2021. In anticipation of the supply issue with the Varenicline Pfizer brand, PHARMAC had listed another brand (Champix, also made by Pfizer) however, there is now a global stock issue affecting both brands. There is no other brand of varenicline available in New Zealand. Prescribers are advised not to prescribe varenicline until the stock issue is resolved and patients already taking varenicline will not be able to fill repeat prescriptions. Patients taking varenicline or seeking help with smoking cessation will need to be advised about alternative funded treatments, e.g. bupropion tablets, nicotine replacement therapy or nortriptyline. Pharmacists should notify patients seeking repeats of their current prescription of the need to return to their prescriber to discuss other treatment options.
You can read more about available treatment options for smoking cessation here: https://bpac.org.nz/BPJ/2015/October/smoking.aspx
New funded medicine option for children with problematic constipation
From 1 July, 2021, sodium picosulfate (Ducolax SP Drops) will be funded
with Special Authority approval as a treatment for children with problematic and refractory constipation if other funded laxatives have been ineffective or not tolerated. Sodium picosulfate is currently unfunded but has been available in New Zealand both as an unapproved medicine prescribed under Section 29 or over-the-counter as a pharmacy-only medicine. It is a stimulant laxative, i.e. increasing intestinal motility to assist with the passage of bowel motions.
Any relevant practitioner may apply for Special Authority funding; the criteria are that the child must:
- Have problematic constipation despite an adequate trial of other oral treatments including macrogol where practical;
and
- Otherwise require a high-volume bowel cleansing preparation or hospital admission
Meningococcal B vaccine to be funded for eligible people
PHARMAC has
announced that meningococcal B vaccine (Bexsero) will be funded from 1 July, 2021 for people who are close contacts
of a confirmed case of meningococcal disease due to any meningococcal group, e.g. A, C, W, Y or B, or who are at higher
risk of meningococcal disease, e.g. people who are immunocompromised. The eligibility criteria were amended slightly
after consultation to also include funding for people to be immunised prior to starting immunosuppressive treatments.
Wider funding for other patient groups is still being considered.
The Ministry of Health’s Immunisation
Handbook will be updated to reflect the eligibility criteria for people to receive funded access to the meningococcal B vaccine.
To see the full list of eligibility criteria, see: https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/decision-2021-06-16-menb-vaccine/

B-SAFE: Atrial fibrillation clinical decision support tool
Can you help us evaluate an electronic decision support tool which has been designed to assist primary
care clinicians with management of atrial fibrillation?
In collaboration with cardiologists and stroke physicians from the National Cardiac and Stroke Networks we have created a tool to assist primary care clinicians
in optimising cardiovascular treatments in patients with atrial fibrillation. The tool has been designed to provide an individualised check-list for treatments known
to decrease the risk of stroke, myocardial infarction, heart failure and bleeding in patients with atrial fibrillation. It has also been designed to
be quick and easy to use as part of routine care.
Click here to view a short video about the tool.
We now wish to evaluate this tool in the ‘Biomarkers for Stroke Prevention in Atrial Fibrillation using Electronic Decision Support (EDS)’
or B-SAFE study in practices that use Medtech-32 or Evolution.
We would greatly appreciate your help with this study. This would involve identifying patients with atrial fibrillation in your practice, gaining
consent from them, and then completing the tool (this should take less than five minutes per patient). You may then adjust the patient's medicine
regimen if you think this is appropriate. We will pay $75 for each patient entered into the study.
If you are interested or would like more information,
please click here.
You can also contact us at b-safe@bestpractice.org.nz
Professor Ralph Stewart, Emeritus Professor Murray Tilyard, Professor Anna Ranta, Professor Richard Troughton
Paper of the week: Potential adverse effects with short-term use of corticosteroids in children
A paper recently published in JAMA
Pediatrics examined the question: "Are there potential harms associated with oral corticosteroid bursts (defined as the use of oral corticosteroids for 14 or fewer days) in children? The conclusion was – Yes.
The study using data from the National Health Insurance Research Database in Taiwan, involved more than one million
children who had received a short-course treatment of oral corticosteroids. The main findings were a 1.4 to 2.2-fold
increase in the risk of gastrointestinal bleeding, sepsis and pneumonia within the first month after taking corticosteroids;
the risk decreased after three months.
Short bursts of oral corticosteroid treatment are commonly used in children with asthma and other respiratory or inflammatory conditions. These findings bring pause to thought of how we assess the risks and benefits of seemingly routine treatments, and how we communicate this to patients and their families.
More on the study findings
- 42% of the approximately four million children (aged < 18 years, average age 9 years) in the national database had at least one oral corticosteroid "burst" within
the five-year study period
- The main reasons for prescribing corticosteroids were acute respiratory tract infections (34%) and allergy
(31%)
- 91% of the children who received corticosteroids did not have a baseline co-morbidity
- Incidence rate differences per 1000 person-years between children administered corticosteroids versus controls
were 0.60 for GI bleeding, 0.03 for sepsis, 9.35 for pneumonia and 0.01 for glaucoma.
- Corticosteroid bursts were significantly associated with a 1.4-fold increase of GI bleeding, 2.0-fold increase
of sepsis, 2.2-fold increase of pneumonia and 0.98-fold increase of glaucoma (i.e. no significant increase in
glaucoma), within the first month after initiation of corticosteroid treatment
- The study did not correct for factors such as antibiotic use or exposure to cigarette smoke
Adverse effects of corticosteroids are usually associated with adults but this study shows that in children they are “not innocuous”. The adverse effects were rare, although potentially serious. Authors conclusion: Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events. The present findings call for a careful re-evaluation regarding the prudent use of corticosteroid bursts in children.
Yao T, Wang J, Chang S. Association of oral corticosteroids bursts with severe adverse events in children. JAMA Pediatr. Online April 19, 2021. doi:10.1001/jamapediatrics.2021.0433
 This Bulletin is supported by the South Link Education Trust
This Bulletin is supported by the South Link Education Trust
If you have any information you would like us to add to our next bulletin, please email:
editor@bpac.org.nz
© This resource is the subject of copyright which is owned by bpacnz. You may access it, but you may not reproduce it or any part of it except in the limited situations described in the terms of use on our website.





