Dabigatran is becoming widely used in general practice in New Zealand since its listing on the
Pharmaceutical Schedule in 2011. In July 2014, approved indications for dabigatran were widened,
and the funded indications now include:
- Prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and a risk factor for stroke
- Primary prevention of venous thromboembolic events in patients undergoing total hip or knee replacement surgery
- Treatment of acute deep vein thrombosis (DVT) and/or pulmonary embolism (PE)
- Prevention of recurrent deep vein thrombosis (DVT) and/or pulmonary embolism (PE)
This report provides an update on the use and monitoring of dabigatran between October, 2013 and September, 2014 in general practice in New Zealand.
 For further information see:
Dabigatran Revisited. BPJ 50 (Feb, 2013)
For further information see:
Dabigatran Revisited. BPJ 50 (Feb, 2013)
Dabigatran in general practice
National Data
22,771
patients taking dabigatran |
32% of patients taking oral anticoagulants are now treated with dabigatran
Of the 71,279 general practice registered patients taking oral anticoagulants (warfarin or
dabigatran) between October 2013 and September 2014, 32% (22,771 people) were dispensed
dabigatran. The proportion of patients recieveing dabigatran has increased from 24% (bpacnz
report, February 2013) to 32% in the last two years.
|
Renal Function
Renal function should be monitored in patients taking dabigatran
Dabigatran is primarily renally excreted so patients must have creatinine clearance >30 mL/min. It should be used with
caution in patients with creatinine clearance between 30 and 50 mL/min,1 and in older patients (aged over 75 years). It is
therefore recommended that renal function is assessed:
- Prior to initiation of dabigatran (baseline testing)
- At least annually for patients aged over 75 years (ongoing monitoring)
- At least annually but ideally three to six monthly in those who have a creatinine clearance of 30 – 50
mL/min (ongoing monitoring)
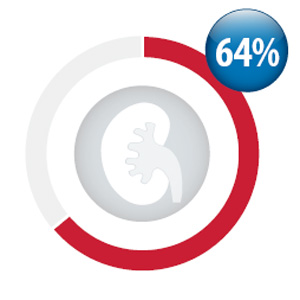 Between October, 2013
and September, 2014, 64%
of patients initiated on
dabigatran (8,242 people)
had a baseline creatinine
test performed (between 3
months prior and 3 weeks
after their first dispensing of
dabigatran )*
Between October, 2013
and September, 2014, 64%
of patients initiated on
dabigatran (8,242 people)
had a baseline creatinine
test performed (between 3
months prior and 3 weeks
after their first dispensing of
dabigatran )*
|
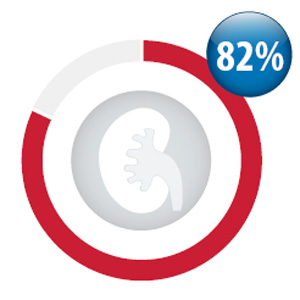 Between October, 2013
and September, 1204, 82%
of patients dispensed
dabigatran (14,529
people) had a creatinine
test performed as part of
ongoing monitoring (within
12 months).
Between October, 2013
and September, 1204, 82%
of patients dispensed
dabigatran (14,529
people) had a creatinine
test performed as part of
ongoing monitoring (within
12 months).
|
Data for your practice
Patients taking dabigatran |
Baseline creatinine testing |
Ongoing monitoring of creatinine |
Total number of your registered patients
dispensed dabigatran:
33
Patients initiated on dabigatran
12
Patients with ongoing dabigatran dispensings
21
|
Percentage of your practice patients
initiated dabigatran with a creatinine test
within three months prior and three weeks
after first dispensing*
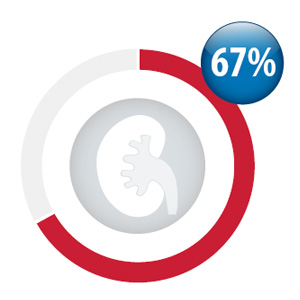
|
Percentage of your practice patients taking
dabigatran with a creatinine test within the
period October, 2013 to September, 2014
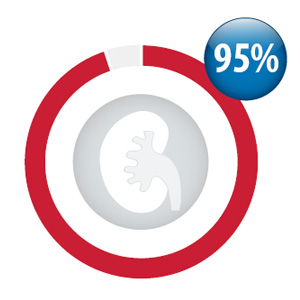
|
Dabigatran Audit
The levels of renal function monitoring in patients taking dabigatran are encouraging. However if you would like to further
audit your practice patients, bpacnz has a clinical audit available to download at: bpacnz.org.nz/audits
* For the purposes of this report, baseline testing was defined as a creatinine test in the three months prior to a
first dispensing of dabigatran, and also included tests performed in the three weeks after to account for patients who collected their
prescription first. Clinicians may have a small number of patients with stable
renal function for whom a creatinine test more than three months prior is considered a satisfactory baseline measure. Some patients may also receive baseline
testing in a hospital setting, which is not included in this report.
1. New Zealand Formulary. 2014. Dabigatran. Available from:
www.nzf.org.nz (Accessed Dec, 2014).