Published: 27th June 2024
Key practice points:
- Antimicrobial resistance (AMR) is a serious and growing global threat which is accelerated by excessive and inappropriate antibiotic use
- New Zealand has previously been identified as having higher rates of antibiotic prescribing than many other developed countries. A key focus in recent years has been on reducing overall rates of antibiotic use and ensuring that when they are required, the appropriate option and regimen is selected based on the specific indication.
- In this analysis, we confirm previous findings identifying a significant decrease in overall antibiotic use between 2019 and 2020 in New Zealand, coinciding with the start of the COVID-19 pandemic. However, we demonstrate that since this decline, dispensing of oral antibiotics has trended back upwards again.
- The overall five-year relative decrease in oral antibiotic dispensing between 2019 – 2023 (-4.5%) matches what would have previously been expected for an annual decrease in the years preceding COVID-19
- The most commonly dispensed oral antibiotic in New Zealand is amoxicillin; dispensing increased after the initial “COVID-era” decline, with 2023 levels matching those seen in 2019
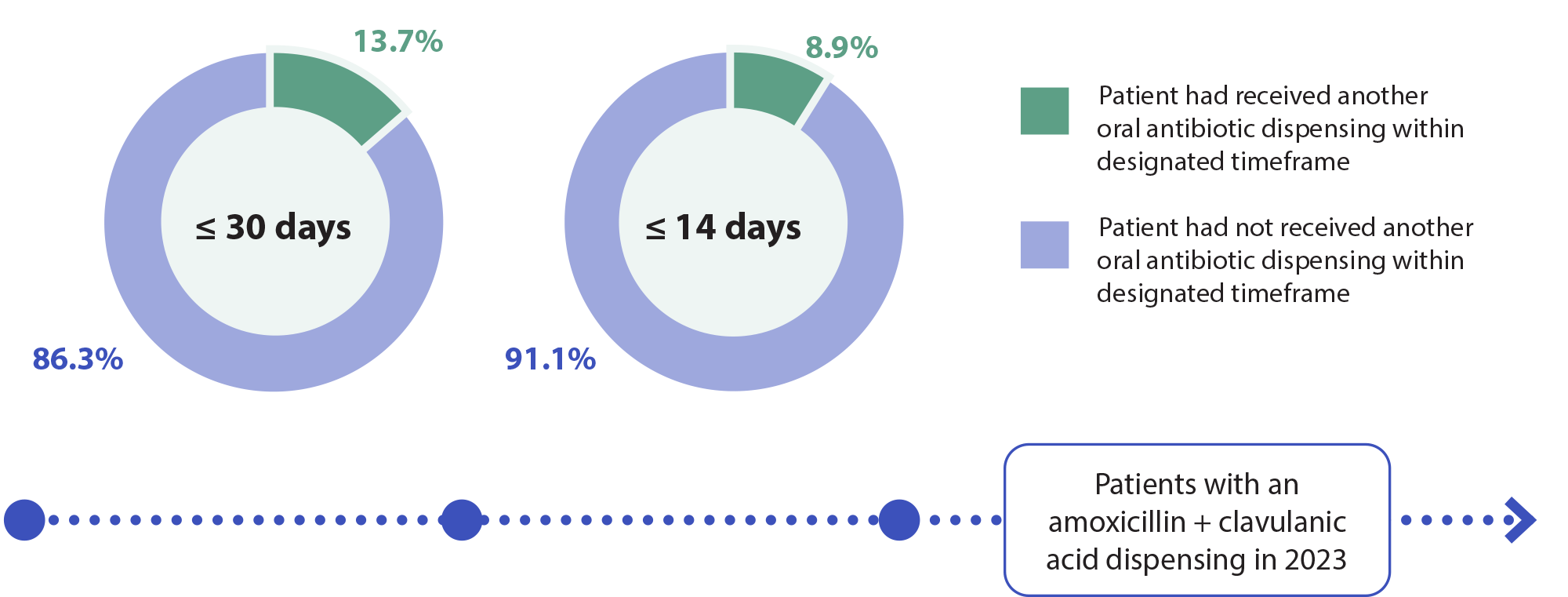
- Amoxicillin + clavulanic acid is the second most commonly dispensed antibiotic, despite having few indications where it is first line. In 2023, > 85% of patients dispensed amoxicillin + clavulanic acid were not dispensed a different antibiotic within the previous 30 days, indicating it was unlikely being used second line.
- Prescribers have shifted their approach to antibiotic selection for uncomplicated urinary tract infection in line with changing recommendations; in 2019, trimethoprim was the most commonly used option, but nitrofurantoin dispensing was significantly higher by 2023
- Topical antibiotic use did not change significantly between 2019 and 2023. In 2023, hydrogen peroxide was dispensed more than fusidic acid.
- Shorter courses of oral antibiotics are emphasised in guidelines (e.g. five to seven days, or less). However, in 2023, almost half of all patients dispensed an oral antibiotic received a supply covering more than seven days.
- There is significant variation in antibiotic dispensing across New Zealand. Pacific peoples, Māori and those living in socioeconomically deprived areas have higher rates of use than other groups.
- Peer comparisons can be effective in reducing the prescribing of antibiotics. If you are a primary care prescriber, see “How does your prescribing compare?” for your personal 2023 prescribing snapshot, including matched comparator and national figures.
The discovery and widespread use of antibiotics represents a pivotal turning point in modern medicine, considerably reducing rates of morbidity and mortality associated with bacterial infections.1 However, the emergence and spread of antimicrobial resistance (AMR) has presented a parallel challenge; resistance is a naturally occurring phenomenon, but it is accelerated by excessive or inappropriate antibiotic use. In 2019, AMR was associated with an estimated 4.95 million deaths worldwide, including 1.27 million cases in which it was considered the direct cause.2 If the issue of AMR is left unaddressed, associated global deaths are predicted to increase to ten million per year by 2050.2
“Unfortunately, unlike COVID-19, many of these deaths by drug-resistant infections are hidden and if documented are usually recorded with a different cause of death, such as cancer or sepsis. If people realised how many deaths were caused by drug-resistant infections across the world they would act as quickly as they have for COVID-19.”
— Professor Laura Piddock
Tackling the drug-resistance pandemic – 2020 and beyond
Concerns around the rising prevalence of AMR, coupled with constraints within the antibiotic-development pipeline, has prompted countries around the world to develop strategies aimed at reducing rates of unnecessary use. Antimicrobial stewardship involves co-ordinated organisational or healthcare system-wide actions, intended to promote and monitor appropriate use of antimicrobials to preserve their effectiveness.3 These efforts must carefully balance the need to maintain antibiotic access when indicated (particularly for groups disproportionately impacted by infection, e.g. Māori and Pacific peoples), against the overall goal of reducing total consumption.4
Antibiotic use in New Zealand prior to the COVID-19 pandemic
New Zealand has higher rates of antibiotic use compared to many other developed countries, with a significant proportion of this likely representing inappropriate use.4 Previous investigation has shown that national community antibiotic dispensing decreased by 13.8% between 2015 and 2018, with an average annual decrease of 4.6% per year.5 The magnitude of decline differed among some regions, but overall antibiotic use decreased across all ethnicities and levels of socioeconomic deprivation.5 As of 2018, community dispensing of beta-lactam antibiotics in New Zealand (including penicillins and cephalosporins) was still twice the rate compared to Scandinavian countries such as Norway and Sweden, and four times the rate seen in the Netherlands.4
Community antibiotic dispensing reduced by more than one-third during the initial months of New Zealand’s COVID-19 response (February to July, 2020);6 this change was not linked to an increase in hospitalisation for sentinel infections that antibiotic use could otherwise prevent.6 These findings suggest that significantly reducing community antibiotic prescribing does not necessarily come at the cost of increased morbidity. Although social distancing, border closures and other transmission-limiting factors will also have likely contributed to the reduction in sentinel infection-associated hospitalisation in this scenario, the trend is reflected in other large international analyses. For example, one UK study encompassing 66 million person-years of follow-up from 671,830 registered patients at 706 general practices between 2002 – 2017 found no association between lower rates of community antibiotic consumption (for any indication) and subsequent risk of serious bacterial infections.7
Clinicians in primary care should continue to reflect on their prescribing decisions, minimising inappropriate antibiotic use to help alleviate the selective pressures driving AMR (see: “Key recommendations for antibiotic prescribing”). For example, with winter approaching, it is timely to reflect on the place of antibiotics for manging respiratory tract infections; in most cases, antibiotic use is not necessary as these types of infections are self-limiting and almost always caused by viruses as opposed to bacteria.
For a patient information sheet on managing seasonal viral illness (without antibiotics), see: bpac.org.nz/2023/flu.aspx
Key recommendations for antibiotic prescribing
- In general, only prescribe antibiotics for bacterial infections if:
- Symptoms are significant or severe; or
- There is a high risk of complications; or
- The infection is not resolving or is unlikely to resolve
- Select the first-line indicated antibiotic at the recommended dose and duration. For general recommendations on community antibiotic prescribing by indication, see the bpacnz Primary Care Antibiotic Guide at bpac.org.nz/antibiotics/guide.aspx. Follow local guidelines/advice where available as resistance patterns may differ across New Zealand.
- Reserve broad spectrum antibiotics for indicated conditions only
- Document the indication on the prescription
- Educate patients about responsible use of antibiotics, including when an antibiotic is not indicated, and the importance of adhering to the advised regimen (dose and duration). Discuss ways that palatability issues or minor adverse effects can be minimised and tips for remembering to take doses on time. For example information sheets, see “Advice if you are prescribed an antibiotic” and “Advice if your child is prescribed an antibiotic”
For further information, see: bpac.org.nz/2018/antibiotics.aspx
The current analysis considers antibiotic use in New Zealand between 2019 and 2023, encompassing the lead up and initial stages of the COVID-19 pandemic. Given the reduced rates of antibiotic use known to have occurred at the beginning of this timeframe (for example, see: bpac.org.nz/report/2020/national-dispensing.aspx), an important focus of the present analysis was whether this trend has continued or whether antibiotic dispensing has returned to “pre-COVID” levels.
Methodology
- The data reported here is based on two Ministry of Health, Manatū Hauora, National Collections:
- The Pharmaceutical Collection holds claim data submitted by pharmacies for all community dispensings of funded medicines
- The Primary Health Organisation Enrolment Collection holds patient enrolment data for patients registered in New Zealand general practices
- bpacnz has no control over data errors which may occur at the pharmacy, Sector Services or Ministry of Health. Discrepancies may also exist due to rounding.
- Data has been excluded for patients who are not registered to a practice in the PHO Enrolment Collection. Registered patients accounted for 99% of pharmaceutical dispensings recorded in the National Collections. Data has also been excluded when a National Health Index (NHI) number was not recorded for the patient.
- The reporting timeframe for any given year encompasses data from the 12 months between 1st January and 31st December
- Data for oral antibiotic use was obtained by combining Therapeutical Group Level 2 categories for “Antibacterials” and “Urinary Tract Infections”, excluding injectable antibiotic formulations
The big picture: overall oral antibiotic use trending upwards since the initial “COVID-19 decline”
Overall oral antibiotic use in New Zealand decreased by almost 20% between 2019 and 2020 (Figure 1), consistent with trends shown in previous investigations highlighting a COVID-19 era-associated drop in dispensing.6, 8 However, rates of antibiotic dispensing then rebounded; by 2023, they were only 4.5% lower than 2019 levels. As such, the overall relative decrease during this five year period (2019 – 2023) matches what would have previously been expected in a single year during the pre-COVID era.5
Overall topical antibiotic use remained relatively stable between 2019 – 2023 (Figure 1). There was a slight decrease in topical antibiotic dispensing between 2019 and 2022, however, levels rose again in 2023 (the absolute difference versus 2019 is negligible).
Figure 1. Overall dispensing of oral and topical antibiotics in New Zealand between 2019 – 2023 (number of dispensed prescriptions per 1,000 registered patients). Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
Amoxicillin remains the most frequently used antibiotic
Amoxicillin is the most commonly dispensed antibiotic in New Zealand, followed by amoxicillin + clavulanic acid and flucloxacillin (Figure 2A; also see: “Amoxicillin + clavulanic acid widely used despite few first-line indications”). The dispensing of many oral antibiotics decreased between 2019 and 2020, and remained low until 2023 (or at least did not exceed 2019 levels). However, there were notable increases in dispensing for both amoxicillin and cefalexin between 2020 and 2023, with cefalexin surpassing doxycycline as the fourth most commonly used oral antibiotic by 2023. Use of cefaclor, a previously widely prescribed cephalosporin, halved between 2019 and 2023 (data not shown).
Of the two topical antibiotics available in New Zealand, fusidic acid is the most commonly prescribed (the other being mupirocin; Figure 2B). Rates of fusidic acid use decreased by 11.6% between 2019 and 2023. The overall magnitude and rate of decline for hydrogen peroxide use (an antiseptic alternative to topical antibiotics) matched that of fusidic acid between 2019 and 2022. However, unlike fusidic acid, dispensings of hydrogen peroxide then increased in 2023 to be almost equivalent to 2019 levels, making it the most commonly used topical treatment prescribed for skin infections. This represents a positive shift in clinical practice, as most skin infections do not require antibiotics, and a topical antiseptic is generally more appropriate to limit the development of AMR (if skin hygiene measures alone are insufficient for control).
For further information on topical antibiotics for skin infections, see: bpac.org.nz/2017/topical-antibiotics-1.aspx and bpac.org.nz/2017/topical-antibiotics-2.aspx
Figure 2. Most commonly dispensed individual (A) oral and (B) topical antibiotic/antiseptic options in New Zealand between 2019 – 2023 (number of dispensed prescriptions per 1,000 registered patients). Note the different scales on the y-axis between panels A and B. Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
Prescribers have changed their approach to antibiotic selection for UTIs
Previously, trimethoprim was considered the first-line empirical antibiotic treatment for adults with uncomplicated urinary tract infection (UTI; cystitis). However, in recent years there has been increasing trimethoprim resistance throughout the country, with over one-quarter of Escherichia coli isolates tested by community laboratories between 2016 – 2018 lacking sensitivity.12, 13 In comparison, < 1% of E. coli tested at this time were resistant to nitrofurantoin, suggesting it is a more suitable first-line empiric option.12, 13 In 2020, a modified-release formulation of nitrofurantoin was funded for the treatment of uncomplicated UTIs;14 dosing is twice daily, as opposed to four times daily with the standard release formulation, making it a more acceptable option. Alternatives to nitrofurantoin may still be required in certain cases, e.g. patients with creatinine clearance < 60 mL/min (e.g. cefalexin or trimethoprim) or with suspected pyelonephritis (e.g. cefalexin).
In our present analysis, we determined that prescribers in New Zealand have adjusted their approach to UTI management in accordance with changing evidence and recommendations (Figure 3). Between 2019 and 2023, trimethoprim dispensing declined by 50% while nitrofurantoin dispensing increased by 60% and it became the most commonly used antibiotic for the treatment of UTIs in New Zealand.
For further information on managing adult UTIs, see: bpac.org.nz/2021/uti.aspx
Figure 3. Most commonly dispensed antibiotics for urinary tract infections in New Zealand between 2019 – 2023 (number of dispensed prescriptions per 1,000 registered patients). Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
N.B. Trimethoprim is also indicated as a first-line option for the management of patients with bacterial prostatitis; this indication may account for a small proportion of the dispensings presented.
Almost half of antibiotic prescriptions were for more than seven days
There has been increased emphasis over time on prescribing shorter durations of antibiotic treatment.15, 16 In most cases, community-acquired infections can be managed using five to seven day antibiotic courses (or shorter), with the reasoning being:15, 16
- Evidence shows that short courses of antibiotics are usually at least as safe and effective as longer courses
- Longer courses may cause additional harm, including adverse effects, disruption of commensal bacteria and promotion of AMR resistance through prolonged antibiotic exposure
In 2023, 36.8% of patients enrolled with a PHO in New Zealand received an oral antibiotic dispensing; 1.8% less than in 2019 (38.6%). Findings from the present analysis show that:
- Dispensed prescriptions per patient. Most patients dispensed oral antibiotics in 2023 only received a single prescription (Figure 4A). Approximately 20% of patients received three or more prescriptions. There were no substantial changes in the number of dispensed prescriptions per patient in 2023 versus 2019.
- Duration of supply. In 2023, 43% of patients receiving an oral antibiotic were dispensed a 6 – 7 day supply, and 9% were dispensed a supply covering ≤ 5 days (Figure 4B). The remaining 48% of patients dispensed an oral antibiotic were provided with a supply of > 7 days. Slightly more patients received a > 10 day supply in 2023 versus 2019, and slightly less received an 8 – 10 day supply. The overall average duration of oral antibiotic supply in 2023 was 11.3 days (data not shown); however, this includes a number of patients prescribed long-term courses of antibiotics (e.g. ≥ 60 or ≥ 90 day supplies) which likely represent indications outside of “routine community antibiotic prescribing”, e.g. long courses for antibiotic prophylaxis, use of doxycycline as an antimalarial or for acne, immunocompromised people with long-term infections. If the data analysis is limited to only include patients dispensed a 1 – 30 day supply of antibiotics, the average duration decreases to 8.5 days, and among patients dispensed a 1 – 15 day supply the average duration is 7.4 days (data not shown). This average for the revised 1 – 15 day analysis is closer to the ideal antibiotic treatment duration, but there is still room for improvement as guidelines support shorter antibiotic courses.
Figure 4. A) Number of oral antibiotic prescriptions dispensed per patient; and B) duration of oral antibiotic supply in 2019 versus 2023 in New Zealand. In both cases, figures expressed as percentage of total patients dispensed an oral antibiotic. Note the different scales on the y-axis between panels A and B. Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
A closer look at national antibiotic use: insights from subgroup analyses
By age group (Figure 5) – during childhood, oral antibiotic use is highest between ages 0 – 4 years (with amoxicillin accounting for 60% of the total use in this age group). Levels then decline as children progress towards adolescence, remain at a lower level through until middle age, before rising again in patients aged over 50 years. Peak oral antibiotic dispensing occurs in patients aged 85 years and over. However, rates of oral antibiotic use in older groups were lower in 2023 than they were in 2019.
Figure 5. Oral antibiotic use by age group in New Zealand in 2019 and 2023 (number of dispensed prescriptions per 1,000 registered patients). Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
By ethnicity (Figure 6) – Rates of oral antibiotic use declined across all ethnic groups between 2019 and 2023. However, Pacific peoples and Māori had higher rates of oral antibiotic dispensing compared with other ethnic groups: in 2023, 44.1% of Pacific peoples and 40.5% of Māori received a dispensing compared to 36.7% of people of European/Other ethnicity. A possible explanation for these differences includes the higher burden of infectious diseases in Pacific peoples and Māori (due to the health inequities experienced by these groups), which results in a greater need for antibiotics and therefore more dispensing. Oral antibiotic use was lowest among Asian patients (30.4%) in 2023, which may reflect the reduced rates of general practice visits reported in this group.17
Figure 6. Oral antibiotic use by ethnicity in New Zealand in 2019 and 2023 (number of dispensed prescriptions per 1,000 registered patients). Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
By region (Figure 7) – there was significant variation in oral antibiotic dispensings between New Zealand regions. For example:
- Counties Manukau had the highest rates of oral antibiotic use in New Zealand for 2023, although use declined by 5.5% since 2019 (when it also had the highest levels of dispensing)
- Oral antibiotic dispensing decreased in most regions between 2019 and 2023; exceptions included Hutt Valley (+1.7%), Wairarapa (+3.6%) and West Coast (+8.6%)
- Oral antibiotic dispensing was consistently lower on average in the South Island compared with the North Island. Of the five South Island regions, all were below the national average in 2019 and 2023. This may potentially reflect the different ethnic representation of the populations in the North and South Islands.
Figure 7. Oral antibiotic use by region in New Zealand in 2019 and 2023 (number of dispensed prescriptions per 1,000 registered patients). Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
By deprivation status (Figure 8) – oral antibiotic dispensing becomes more prevalent with increasing socioeconomic deprivation. In 2023, there were 148 more dispensed oral antibiotic prescriptions per 1,000 registered patients in the most deprived socioeconomic quintile compared with the least deprived quintile (relative difference = 20%). However, this magnitude of difference was slightly smaller than it was in 2019 (25%).
Figure 8. Oral antibiotic use by socioeconomic deprivation quintile in New Zealand in 2019 and 2023 (number of dispensed prescriptions per 1,000 registered patients). Data obtained from Ministry of Health, Manatū Hauora, Pharmaceutical collection, 2024.9
Amoxicillin + clavulanic acid widely used despite few first-line indications
The high rates of amoxicillin + clavulanic acid use identified in Figure 2A is notable as there has been increasing emphasis in recent years on avoiding excessive use (mainly to reduce the emergence of AMR).10 There are limited indications where amoxicillin + clavulanic acid is first line (e.g. mammalian bites, diabetic foot infections), but it is a second-line option across several indications.11 It is sometimes favoured by clinicians as a way of “covering” a wide range of clinical possibilities due to its broad spectrum of activity.10 However, further analysis of the data does not support “second-line use” as an explanation for the high rates of dispensing (see below).
Further analysis. In 2023, only 8.9% of patients who were dispensed amoxicillin + clavulanic acid received a dispensing for a different oral antibiotic within the previous 14 days. When this timeframe is expanded to the previous 30 days, the figure only rises to 13.7%.* This data indicates that a substantial proportion of amoxicillin + clavulanic acid prescribing is due to its use as a first-line, rather than second-line, choice. This is out of step with the principles of AMR and guidance on antibiotic choices.
Subgroup analysis for 2023 amoxicillin + clavulanic acid data shows:
- Dispensing trends when assessed by ethnicity, region and socioeconomic deprivation match those seen for total oral antibiotic use (Figures 6 – 8), i.e. use is highest among Pacific peoples/Māori, patients within the most deprived socioeconomic quintile and those living in most North Island regions
- When assessed by age group, rates of dispensing are low during childhood and adolescence, but then progressively increase from early adulthood and peak in patients aged 85 years and over. This differs from the trend observed for total/overall oral antibiotic use (Figure 5), where there are high levels of dispensings in patients aged 0 – 4 years, followed by a trough, which only begins to increase notably in patients aged over 50 years.
*The indication/diagnosis is not included with dispensing data, therefore these timeframes and parameters were selected to broadly encompass a usual period of antibiotic treatment decision making relating to a single indication. However, we acknowledge this is an imperfect measure of second-line use. For example, this approach may also include second line amoxicillin + clavulanic acid dispensing for a recurrent infection originally treated > 30 days ago.
The bottom line
Since a significant decline in oral antibiotic dispensing in New Zealand at the start of the COVID-19 pandemic, rates of use then trended back upwards. As of 2023, oral antibiotic use was only 4.5% lower than in 2019, and up almost 20% on the 2020 trough. It is currently unclear whether overall use will continue to increase, stabilise or decline moving forward.
These findings indicate a clear need to renew efforts to reduce inappropriate antibiotic use in New Zealand, particularly considering that research shows that modest decreases in overall prescribing does not substantially compromise patient safety (at a population level).6, 7 All prescribers can do their part to optimally manage infections for individual patients, however, true progress in antimicrobial stewardship requires unified system-level action. This is particularly important given variable rates of prescribing noted in this analysis across different regions, ages, ethnic and socioeconomic groups.
Duration of supply should be a key focus for prescribers given that almost half of all patients dispensed an oral antibiotic in 2023 received a supply covering more than seven days. Although longer antibiotic courses are indicated in select cases (e.g. management of chronic wet cough, prevention of streptococcal throat infections/rheumatic fever, infections occurring in sites where antibiotic penetration or immune responses are relatively poor), the vast majority of patients with community-acquired infections should be managed using shorter antibiotic courses (i.e. five to seven days, or shorter). The potential success of short-term antibiotic regimens can be increased by educating patients on the importance of adherence.18
For further discussion around appropriate durations for antibiotic treatment, see: bpac.org.nz/2018/antibiotics.aspx
If you are a primary care prescriber and have a My bpac account, log in to see your personalised prescribing.
If you don’t have a My bpac account,
sign up for free.
