Published: 24th January, 2025
Key practice points:
- The presentation of people with heart failure in primary care can vary substantially, ranging from mild and non-specific symptoms (e.g. a reduced exercise capacity and malaise), through to those with classical key features (e.g. ankle swelling, shortness of breath, orthopnoea)
- Clinical examination can help to identify more specific signs of heart failure, e.g. elevated jugular venous pressure (JVP), positive abdominojugular reflux, S3 (gallop rhythm) and a laterally displaced apical impulse; however, the absence of these features does not exclude the possibility of heart failure
- The patient’s history should be reviewed to:
- Identify whether they have made behavioural changes to compensate for symptoms, e.g. reducing physical activity in response to shortness of breath
- Assess for other factors that may be an underlying cause or exacerbate symptoms, e.g. co-morbidities (such as arrhythmias or ischaemia) or concomitant medicine use
- If heart failure is still suspected, perform an ECG to check for any obvious underlying abnormalities (e.g. atrial fibrillation, ischaemia) and request a brain natriuretic peptide (BNP) level. Additional investigations can then be considered depending on patient-specific factors, e.g. chest X-ray may be useful to confirm fluid overload and to exclude other causes of dyspnoea.
- A clinical diagnosis can be made if patients have symptoms/signs of heart failure and are not “ruled-out” based on having a low BNP result. Referral for an echocardiogram helps to refine long-term treatment decisions, however, it is not required to make an initial diagnosis.
- Echocardiography can distinguish the type of heart failure, and is particularly useful for identifying associated cause(s); this in turn can guide some treatments (e.g. medical, surgical and devices). The three classifications of heart failure are:
- Heart failure with reduced ejection fraction (HFrEF) – patients with reduced left ventricular ejection fraction (LVEF) of ≤ 40%
- Heart failure with mildly reduced (or “mid-range”) ejection fraction (HFmrEF) – patients with a LVEF of 41 – 49%
- Heart failure with preserved ejection fraction (HFpEF) – patients with a LVEF of ≥ 50%, and evidence of relevant structural heart disease, and/or diastolic dysfunction with a high filling pressure
- If the patient has significant or distressing symptoms at presentation, or an acute cardiac condition is suspected to be causing heart failure (e.g. acute coronary syndrome), consider urgent secondary care referral where further management decisions will be made. Treatment for patients initially managed in primary care is guided by the severity of symptoms, the presence and type of co-morbidities and relevant laboratory investigations.
Heart failure affects 2 – 3% of all adults in New Zealand, and ≥ 10% of those aged over 70 years.1, 2 The associated burden is projected to increase over time due to an ageing population as well as increased prevalence of risk factors, e.g. obesity, sedentary lifestyles, type 2 diabetes and ischaemic heart disease.3 An estimated one in five residents in aged residential care facilities have heart failure,4 but it is also becoming more common in younger age groups.1
Māori and Pacific peoples in particular are disproportionately affected by heart failure. Onset typically occurs at a younger age and rates of hospitalisation and mortality are substantially increased in Māori compared with non-Māori (at least four- and two-fold higher, respectively).5, 6 These disparities have widened over time. Between 2006 – 2018, hospitalisation rates for heart failure decreased among older Europeans, but rates remained unchanged among Māori and Pacific peoples.5 Māori and Pacific peoples aged < 50 years are six times more likely to be hospitalised for heart failure than Europeans in the same age group.5
Most patients with heart failure require secondary care input at some point. The five-year survival rate is < 50% following diagnosis,3 and < 37% following first hospitalisation.7 This prognostic outlook is improving with more widespread use of guideline-directed medical therapy (see: “Part 2 - Initiating and optimising treatment for heart failure”), but remains worse than for many common cancers. As such, there is a clear need for clinicians to identify potential cases as early as possible, so that effective pharmacological treatment can be initiated. However, making a diagnosis of heart failure in primary care can be challenging; it is largely a clinical diagnosis relying on presenting features in the context of the patient’s history, initially supported by laboratory markers (see: “Request brain natriuretic peptide (BNP) level”), and later by echocardiography findings (see: “Refer for an echocardiogram but do not delay treatment”).
Identifying heart failure in primary care can be difficult as presentation varies between patients due to differences in the underlying pathology and compensatory mechanisms (Table 1).3, 8 In addition, symptoms and signs may only be mild at first, have a gradual onset or be difficult to interpret, e.g. in patients with multiple co-morbidities. As a result, recognising heart failure often relies on the clinician determining whether the combination of symptoms and signs a patient is presenting with warrants further investigation based on their clinical history (see: “Piecing together the puzzle: patient history”).
Non-specific symptoms such as dyspnoea, fatigue or reduced exercise tolerance may not immediately raise suspicion of heart failure as these can be associated with other conditions, e.g. COPD. Heart failure becomes a more likely diagnosis if these symptoms and signs occur with other features of fluid overload, such as lower limb swelling, rapid weight gain or specific types of dyspnoea, e.g. in a recumbent position (orthopnoea) or that awakens a patient from sleep (paroxysmal nocturnal dyspnoea).8, 9 More specific signs may be identified during clinical examination, e.g. elevated jugular venous pressure (JVP), positive abdominojugular reflux, S3 (gallop rhythm) and a laterally displaced apical impulse.8, 9 However, these features can be difficult to detect accurately, and their absence does not exclude the possibility of heart failure.9
Table 1. Symptoms and signs of heart failure.3, 8, 9
|
Symptoms |
|
Signs |
| More typical |
- Dyspnoea (often on exertion)
- Orthopnoea
- Paroxysmal nocturnal dyspnoea
- Reduced exercise tolerance
- Excessive fatigue
- Weakness
|
More specific |
- Elevated jugular venous pressure
- Hepatojugular reflux
- Third heart sound (S3 gallop rhythm)
- Laterally displaced apical impulse
|
| Less typical |
- Nocturnal cough
- Malaise
- Wheezing
- Bloating
- Anorexia
- Confusion (especially in older patients)
- Depression
- Palpitations
- Dizziness
- Syncope
|
Less specific |
- Weight gain (> 2kg/week)
- Weight loss and cachexia in patients with severe heart failure
- Peripheral oedema, e.g. ankle swelling
- Bibasilar crackles
- Cardiac murmur
- Tachycardia
- Tachypnoea
- Hepatomegaly
- Cheyne-Stokes respiration
- Ascites
|
Considering the patient’s history provides context for the presenting features if heart failure is suspected and clues regarding the likely cause(s).
 Ask about behavioural or lifestyle changes that have been made to avoid symptoms. Emerging features of heart failure can be subtle or unintentionally disguised by patients making behavioural or lifestyle changes to compensate, e.g. limiting physical activity to avoid shortness of breath and therefore dyspnoea is not reported as a symptom.8 Patients may not be aware that they have made these changes until they are specifically asked about them.
Ask about behavioural or lifestyle changes that have been made to avoid symptoms. Emerging features of heart failure can be subtle or unintentionally disguised by patients making behavioural or lifestyle changes to compensate, e.g. limiting physical activity to avoid shortness of breath and therefore dyspnoea is not reported as a symptom.8 Patients may not be aware that they have made these changes until they are specifically asked about them.
 Identify contributing factors and co-morbidities that are inadequately controlled. In some cases, co-morbidities may be the underlying cause of heart failure; their presence can add to clinical confidence regarding the diagnosis, and if their management can be optimised then the risk of acute decompensation (new or worsening symptoms and signs) may reduce.8 For example, at least 90% of people with heart failure have a history of hypertension and approximately 40% have diabetes.3
Identify contributing factors and co-morbidities that are inadequately controlled. In some cases, co-morbidities may be the underlying cause of heart failure; their presence can add to clinical confidence regarding the diagnosis, and if their management can be optimised then the risk of acute decompensation (new or worsening symptoms and signs) may reduce.8 For example, at least 90% of people with heart failure have a history of hypertension and approximately 40% have diabetes.3
Contributing factors and conditions associated with heart failure include:3, 8, 10
- Hypertension
- Ischaemic heart disease
- Valvular disease
- Cardiomyopathies, e.g. diabetic, hypertrophic, alcohol-related, genetic*
- Arrhythmias, e.g. atrial fibrillation
- Obesity
- Thyrotoxicosis (causing high output failure)
- Chronic kidney disease or worsening renal function
- COPD
- Obstructive sleep apnoea
- Anaemia
- Smoking
- Infection (leading to myocarditis or cardiomyopathy)
- Drug-induced, e.g. prior use of cardiotoxic chemotherapy, methamphetamine
* An estimated 25 – 40% of people with dilated cardiomyopathy and a positive family history have an underlying genetic cause10
 Consider current medicine use and whether adjustments can be made. Given that most patients with heart failure have co-morbidities, it is likely they are already taking other medicines. Polypharmacy is common in this context, and is associated with increased mortality risk.11 Numerous medicines can influence cardiac functioning – these may increase the risk of heart failure developing, or worsen outcomes for patients with established disease.8 Examples include:8, 10, 12
Consider current medicine use and whether adjustments can be made. Given that most patients with heart failure have co-morbidities, it is likely they are already taking other medicines. Polypharmacy is common in this context, and is associated with increased mortality risk.11 Numerous medicines can influence cardiac functioning – these may increase the risk of heart failure developing, or worsen outcomes for patients with established disease.8 Examples include:8, 10, 12
- NSAIDs – can cause renal impairment and sodium and water retention, increased vasoconstriction, and an impaired response to diuretics/angiotensin-converting enzyme (ACE) inhibitors
- Corticosteroids – can cause sodium and water retention
- Thiazolidinediones, e.g. pioglitazone – can cause dose related fluid retention; avoid use in patients with heart failure
- Most calcium channel blockers (with the exception of amlodipine and felodipine) – can cause negative inotropic effects such as weakening cardiac muscle contraction and slowing heart rate
- Antiarrhythmic medicines, e.g. sotalol and disopyramide can prolong the QT interval, flecainide can cause negative inotropic effects
- Some antidepressants, e.g. tricyclic antidepressants, citalopram, venlafaxine – can cause a range of adverse cardiovascular effects, including bradycardia, tachycardia, hypertension
As a chronic and progressive condition, most patients with heart failure are seen in secondary care at some stage – whether it be for echocardiography to refine primary care management or for cardiologist review. However, people presenting to primary care with the following features should be more urgently referred (or immediately sent to the emergency department):8, 9
- Significant or distressing symptoms of orthopnoea, paroxysmal nocturnal dyspnoea, syncope, ischaemic chest pain
- An acute cardiac condition is suspected to be the cause of heart failure, including fast atrial fibrillation or other sustained arrythmias (e.g. tachycardia, bradycardia), a significant new heart murmur or acute coronary syndrome
- Newly diagnosed or worsening heart failure during pregnancy
Defining heart failure
The terminology used to describe heart failure has changed over time:
Most recent guidelines now distinguish an intermediate subtype of heart failure with mildly reduced (or “mid-range”) ejection fraction – HFmrEF.10, 13, 14 Resources that do not include this subtype use a HFrEF LVEF threshold of < 50%.8 The same treatment approach is generally recommended for patients with HFmrEF and HFrEF, albeit with less convincing evidence of an absolute benefit for those with HFmrEF. A fourth subtype, heart failure with an improved ejection fraction (HFimpEF), has also been proposed for patients with a previous LVEF ≤ 40% who subsequently have a follow-up measurement of LVEF > 40%.10 Despite its usefulness in guiding treatment decisions, classification using LVEF has some limitations, including high intra- and inter-observer variability and acute load-dependent fluctuations.
Heart failure is a progressive condition involving an impaired ability of the heart to fill with blood (at a normal pressure) or eject blood sufficient to meet the requirements of metabolising organs.8 This can result from various pathological processes that impact cardiac or structural and/or function, including coronary artery disease and myocardial infarction, valvular conditions, heart rhythm/conduction abnormalities, myocarditis or cardiomyopathies (either acquired or genetic).13 These factors trigger compensatory mechanisms to initially maintain cardiac output and systemic demands, including the activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system – collectively referred to as “neurohormonal activation”.13 However, over time these compensatory mechanisms begin to fail and maladaptation results, contributing to features such as fluid retention, vasoconstriction, myocardial stress, cardiac remodelling, hypertrophy and interstitial fibrosis.13
Disease progression can differ between patients, with some experiencing prolonged periods without symptoms before clinical deterioration becomes apparent and a diagnosis is made. Heart failure staging has been proposed by the American College of Cardiology (ACC)/American Heart Association (AHA) to emphasise its development and progression (Figure 1).10 In patients with symptomatic disease (i.e. Stages C and D), the functional impact can be distinguished using the NYHA classification system (see: “Determining the patient’s baseline functional status (NYHA Class)”). Early detection of heart failure and initiation/optimisation of treatment is essential to alleviate the decline in clinical status and quality of life for patients, while also reducing hospitalisation due to episodes of acute decompensation. In patients with HFrEF, this can be achieved using guideline-directed medical therapy (GDMT) – otherwise referred to as the “four pillars” of heart failure treatment: an angiotensin receptor-neprilysin inhibitor (ARNI), beta blocker, mineralocorticoid receptor antagonist (MRA) and a sodium-glucose co-transporter-2 (SGLT-2) inhibitor.
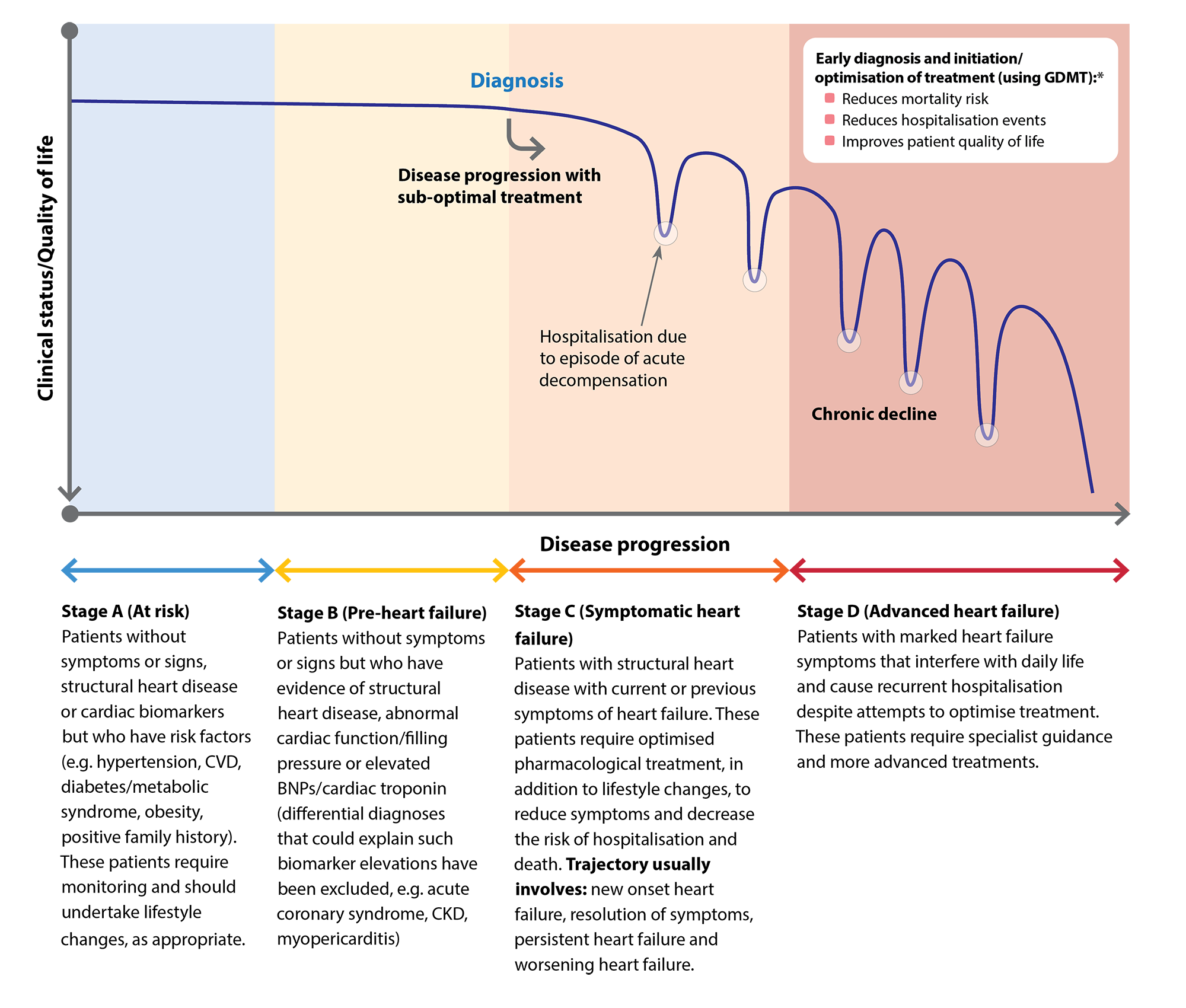
Figure 1. Heart failure disease progression. Adapted from Steinberg et al 2017 and AHA/ACC/HFSA 2022.10, 14
* GDMT = guideline-directed medical therapy – otherwise referred to as the “four pillars” of heart failure treatment
For further information on managing patients with heart failure, including the importance of GDMT, see: “Part 2 - Initiating and optimising treatment for heart failure “
Perform an ECG
When heart failure is suspected, perform an ECG to identify any underlying cardiac abnormalities (e.g. left bundle block, poor R wave progression, ischaemic changes, atrial fibrillation) or other rhythm disturbances as these are common causes of heart failure.8, 10 Ventricular arrhythmias are responsible for a high proportion of sudden deaths in people with heart failure and the presence of significant dysfunction may influence the urgency of secondary care involvement.10
Practice point: If the patient has reported acute chest pain and the ECG reveals a ST-segment abnormality – particularly ST-segment elevation – co-ordinate urgent hospital transfer, alert the on-call cardiologist or emergency department consultant, and provide appropriate acute treatment while awaiting transfer (see: bpac.org.nz/BPJ/2015/April/coronary.aspx and bpac.org.nz/BPJ/2015/August/coronary.aspx).
Request brain natriuretic peptide (BNP) level
Laboratory assessment of BNP levels should be requested for any patient with a pattern of symptoms and signs indicating possible heart failure. BNP is a hormone produced by cardiomyocytes in response to increased ventricle wall tension, which is associated with natriuretic, diuretic and vasodilatory effects (thereby countering the effects of sympathetic nervous system and RAAS activation).15, 16 Following synthesis, the BNP prohormone is cleaved and secreted into the blood in equimolar amounts (1:1 ratio), including:15, 16
- The biologically active BNP-32 hormone; and
- The inactive NT-proBNP portion
Both BNP biomarkers can be used as a proxy for how “stressed” the heart is; the higher the levels, the more likely a diagnosis of heart failure.8 However, NT-proBNP testing has several advantages over BNP-32, including a longer half-life, greater stability and levels that are not influenced by heart failure medicines (e.g. the neprilysin inhibitor sacubitril prevents BNP-32 degradation but does not significantly influence NT-proBNP).16 In most cases, clinicians in New Zealand cannot specify the type of BNP test on a laboratory form, and there is regional variation in which test will be done (i.e. BNP-32 or NT-proBNP).
Interpreting BNP results
BNP testing is considered primarily to be a “rule out” test, as other conditions can also affect levels.8, 10 For example, increased BNP levels may be present in people with atrial fibrillation, COPD, severe renal impairment, post-myocardial infarction, or in those with left ventricular hypertrophy.8, 10, 15 Decreased BNP levels may occur in people with obesity, hypothyroidism or who are taking certain medicines (such as diuretics, vasodilators or ACE inhibitors).8, 10, 15 N.B. A decreased BNP in patients with heart failure taking diuretics or other heart failure medicines usually correlates with a favourable change in volume status. However, serial BNP monitoring is not routinely recommended post-diagnosis unless it will affect management decisions (see: “Part 2 - Initiating and optimising treatment for heart failure”).
For patients with “intermediate” BNP readings, consider these potentially confounding factors within the context of the full clinical picture to determine whether a diagnosis of heart failure should be made.
Only at very high levels is BNP considered to be a “rule in” test for heart failure and for NT-proBNP this threshold is age dependent. Table 2 outlines the diagnostic thresholds used in 2018 Australasian guidelines, however, “normal ranges” are generally provided alongside laboratory results. Many laboratories in New Zealand report BNP results in units of pmol/L, but pg/mL (or ng/L) is more commonly used in international literature.
Patients with normal BNP-32 or NT-proBNP levels are very unlikely to have heart failure, and a differential diagnosis should be considered.8
Table 2. General BNP diagnostic thresholds for heart failure based on Australasian guidelines and used in most New Zealand laboratories.*† N.B. Diagnostic thresholds may differ between different international guidelines and laboratories.
|
BNP component tested
|
BNP-32 |
NT-proBNP |
|
Unlikely (rule-out)
|
< 30 pmol/L (100 pg/mL)
|
< 35 pmol/L (300 pg/mL)
|
|
Possible
– consider in context of full clinical picture
|
Patients with measurements that fall within this intermediate area who also present with symptoms and/or signs with a good specificity for heart failure, e.g. paroxysmal nocturnal dyspnoea and/or elevated venous jugular pressure, are likely to have heart failure
|
|
Likely (rule-in)
|
> 116 pmol/L (400 pg/mL)
|
Age dependent:
< 50 years: > 50 pmol/L (450 pg/mL)
50–75years: > 100 pmol/L (900 pg/mL)
> 75 years: > 210 pmol/L (1,800 pg/mL)
|
* The type of test available and the reference range for BNP may differ between laboratories or regions. If the BNP reference range is not provided by your local laboratory, consider discussing the findings with a clinical biochemist.
† To covert BNP from pmol/L to pg/mL, multiply by 3.47; to convert NT-ProBNP from pmol/L to pg/mL, multiply by 8.46
Additional tests to consider
BNP is the primary biomarker to assess the likelihood of heart failure, however, additional laboratory tests should also be requested to help clarify the diagnostic picture and guide treatment decisions, e.g. full blood count, electrolytes, renal and liver function testing.8, 10 In many cases, it is also reasonable to assess HbA1c and lipid levels as part of a routine cardiovascular disease risk evaluation.8, 10
Depending on the specific patient, other tests can also be considered 8–10
- Thyroid function – hyperthyroidism (or less commonly hypothyroidism) can precipitate heart failure and is a potentially reversible cause of heart failure
- C-reactive protein (CRP) – if infection is suspected
- Serum troponin – if there is acute onset of symptoms or an acute coronary syndrome is possible. N.B. Patients with severe cardiomyopathy or clinical heart failure may have elevated troponin levels;17 this is an adverse prognostic factor but does not necessarily indicate an acute coronary syndrome is present.
- Iron studies (including iron levels, ferritin, transferrin saturation [TSAT]) – if iron deficiency is suspected or risk factors are present. Conventional thresholds for iron deficiency (usually serum ferritin < 20 micrograms/L) are not reliable in patients with heart failure as this condition involves a systemic inflammatory state and ferritin levels become elevated in response to inflammation.18, 19 Iron deficiency can be diagnosed in patients with heart failure if serum ferritin levels are < 100 micrograms/L (absolute iron deficiency), or if serum ferritin levels are < 100 – 300 micrograms/L and TSAT is < 20% (functional iron deficiency).18, 19
- Chest X-ray – not routinely required, but can show enlargement of the heart and pulmonary fluid overload, and help assess possible alternative respiratory causes of dyspnoea
- Spirometry – may be useful to further assess potential respiratory causes of dyspnoea but does not assist in the diagnosis of heart failure
- Other secondary care investigations – if the diagnostic work-up of a patient with suspected heart failure does reveal potential underlying causes, there are various additional investigations, usually performed in secondary care, including stress testing (while performing exercise using a treadmill or stationary bike) or stress echocardiography, coronary angiography, cardiac magnetic resonance imaging (MRI) or computed tomography (CT) angiogram and rarely, cardiac biopsy, and genetic testing in patients with a family history of cardiomyopathy
Determining the patient’s baseline functional status (NYHA Class)
The New York Heart Association (NYHA) functional classification can help in assessing baseline symptom severity and the relative effect of treatment decisions (Table 3).8, 10 Patients are categorised into one of four groups depending on the amount of exertion needed to exacerbate symptoms (Table 3).8, 10 However, there are subjective limitations to this classification system, e.g. variability between clinicians in detecting signs, and interpretation of terms such as “ordinary”, “slight” and “marked” by the patient.
While this classification does not assist in making a diagnosis of heart failure, it can be a useful starting point for informing a discussion around symptoms and expected improvements. In addition, the patient’s NYHA functional class is required as part of the Special Authority application for funding of certain medicines used in the treatment of heart failure, e.g. sacubitril + valsartan, empagliflozin.
Table 3. New York Heart Association functional classification of heart failure.8, 10
|
Class I
|
Asymptomatic – no limitation of physical activity
The patient does not develop undue dyspnoea, fatigue or palpitations with ordinary physical activity
|
|
Class II
|
Mild symptoms – slight limitation of physical activity
The patient is comfortable at rest, but develops dyspnoea, fatigue or palpitations with ordinary physical activity
|
|
Class III
|
Moderate symptoms – marked limitation of physical activity
The patient is comfortable at rest, but develops dyspnoea, fatigue or palpitations with less than ordinary physical activity
|
|
Class IV
|
Severe symptoms – unable to do any physical activity without discomfort
The patient may have symptoms at rest and if any physical activity is undertaken, the level of discomfort is increased
|
Echocardiography is considered the “gold standard” test for supporting a heart failure diagnosis. However, wait times vary substantially across New Zealand, and early intervention is essential to improve prognostic outcomes.8
Therefore, heart failure should be diagnosed clinically first (i.e. based on the history, physical examination, ECG and BNP findings), and treatment initiated immediately under the assumption that the patient has HFrEF (see: “Defining heart failure” and “Part 2 - Initiating and optimising treatment for heart failure“). Long-term management decisions can then be refined once the echocardiogram result is available, if necessary. For example, the echocardiogram findings may reveal abnormalities in the LVEF, left ventricular hypertrophy, systolic and diastolic function, elevated right heart pressures, or evidence of ischaemic damage.9, 20
Identifying potential HFpEF early in primary care
The estimated prevalence of HFpEF is increasing, with some studies now indicating it accounts for up to half of all heart failure cases.3, 21 Various factors might explain this, including advances in diagnostic capabilities, more uniform diagnostic parameters used in studies to estimate prevalence, or the association of HFpEF with age-related co-morbidities. The pathophysiology of HFpEF is less well defined compared with HFrEF, but it generally affects older people with multiple co-morbidities,21 and is more prevalent among females.3 Notably, HFpEF symptom burden at presentation is also often more severe in females versus males, which may influence initial detection.21
However, HFpEF remains under-recognised, particularly as patients can present with a diverse range of co-morbidities, e.g. hypertension, diabetes, obesity.21 It can be challenging (or impossible) to differentiate this subtype based on clinical features alone. The distinction between HFrEF/HFpEF is generally made in secondary care based on echocardiography findings (see: “Defining heart failure”), and treatment normally proceeds assuming that a patient has HFrEF until proven otherwise.
Sometimes a patient can be identified early in primary care as being more likely to have HFpEF (even in the absence of echocardiography results), based on a particular clinical picture of obesity, dyspnoea, exercise intolerance and oedema.21 These patients should be discussed with cardiology more promptly, but only after excluding:21
- Non-cardiac causes of symptoms/signs, e.g. lymphoedema, chronic kidney disease, liver disease, chronic venous insufficiency
- Cardiac mimics of HFpEF, e.g. hypertrophic cardiomyopathy, infiltrative cardiomyopathy, valvular heart disease, pericardial disease. Cardiac amyloidosis is an important mimic to consider, potentially accounting for one in eight suspected HFpEF cases.22 It is associated with misfolded proteins being deposited in various organs, including the heart.23 This condition is frequently missed, with half of patients visiting more than five clinicians before amyloidosis is diagnosed; delays in diagnosis/treatment are associated with early mortality.23 For further information, see: https://www.heartlungcirc.org/article/S1443-9506(24)00123-9/fulltext.
There can also be further challenges during the diagnostic work-up of patients with possible HFpEF. For example, BNP levels are often lower (especially in patients with obesity) and in some cases may be below diagnostic thresholds, and echocardiogram may not demonstrate obvious structural/functional cardiac abnormalities.21
To support the diagnostic evaluation, American guidelines recommend using scoring tools to estimate the likelihood of HFpEF, such as the H2FPEF tool and HFA-PEFF algorithm.21 However these require more in-depth testing information than is routinely available in general practice; H2FPEF requires doppler echocardiography, and HFA-PEFF requires BNP levels, echocardiogram results, diastolic stress testing and specialised investigations (e.g. MRI/CT imaging, biopsy, genetic testing).21
Keep reading: Part 2 – Initiating and optimising treatment for heart failure





