In this report 
View / Download pdf version
of this report
Issues in prescribing dabigatran in general practice
Dabigatran is the first new oral anticoagulant made available for clinical use in over fifty years. Warfarin has been
used for many years but has two major limitations; a narrow therapeutic range and a highly variable dose response. Dabigatran
has a simpler dosing regimen, however, twice-daily dosing is required and no monitoring test is available. This report
will highlight a number of issues that are relevant to General Practitioners when treating patients with dabigatran.
National Data
14483
patients taking dabigatran |
One-quarter of patients taking oral anticoagulants are now treated with dabigatran
Out of 59164 general practice registered patients taking oral anticoagulants (warfarin or dabigatran)
between July 2011 and June 2012, 24% (14483 people) were dispensed dabigatran. |
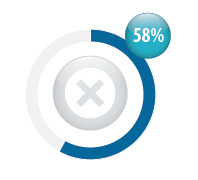 |
It is not recommended that patients who are stable on warfarin are changed to dabigatran
If a patient taking warfarin has stable INR and good venous access, there is no indication to switch them to dabigatran.
In New Zealand, 58% of patients taking dabigtran were previously treated with warfarin (8399 people). |
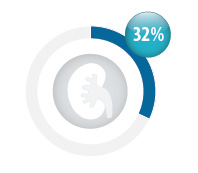 |
Renal function should be checked before a patient is started on dabigatran
Dabigatran is primarily renally excreted so patients must have creatinine clearance >30 mL/min. It should be used
with caution in patients with creatinine clearance between 30 and 50 mL/min,4 and in older patients (aged >80 years).
In New Zealand, only 32% of patients dispensed dabigatran had a creatinine test requested within
the month prior to their first dispensing of dabigatran (4589 people). |
 For correspondence regarding renal function testing for dabigatran, see "Correspondence: Practice report on dabigatran", BPJ 52 (April, 2013).
For correspondence regarding renal function testing for dabigatran, see "Correspondence: Practice report on dabigatran", BPJ 52 (April, 2013).

Be aware of adverse effects and potential drug interactions
The most frequently reported adverse reaction to dabigatran are dyspepsia and other non-haemorrhagic gastrointestinal
symptoms. However, bleeding, as with any anticoagulant medicine, remains one of the main adverse risks of dabigatran.
The risk of bleeding can be minimised by ensuring that INR is < 2 before a patient is changed from warfarin to dabigatran,
that the dose is appropriate for age and renal function, and that dabigatran is not used with medicines that can increase
bleeding risk, e.g. aspirin, clopidogrel, dipyridamole and NSAIDs.
Data for a sample practice
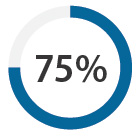
Patients taking dabigatran
Total number of registered patients dispensed dabigatran: |
20 |
Percentage who switched from warfarin: |
 |
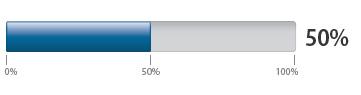
Creatinine tested before starting dabigatran
Percentage of patients with a creatinine test within a month before starting dabigatran: |
 |
References
- Ministry of Health. 2012. Primary Health Organisation Enrolment Collection (Accessed Nov, 2012).
- Ministry of Health. 2012. Laboratory Claims Collection (Accessed Nov, 2012).
- Ministry of Health. 2012. Pharmaceutical Collection (Accessed Nov, 2012).
- New Zealand Formulary. 2012. Dabigatran. Available from: www.nzf.org.nz (Accessed
Nov, 2012).