For updated screening recommendations, see:
"The
annual diabetes review: screening, monitoring and managing complications"
In this article 
View / Download
pdf version of this article
| Key concepts |
- Foot ulceration and damage is a common complication of diabetes
- Feet should be checked at least once per year in every person with diabetes and more regularly in those who are
at higher risk of developing foot complications
- Educate about foot care, appropriate foot wear and foot hygiene at every opportunity
- Refer to, or consult with, a podiatrist, diabetologist or vascular specialist if foot complications develop or
if there are any concerns
|
Diabetes in New Zealand – a growing concern
In 2000 the Ministry of Health acknowledged that diabetes was a major concern in New Zealand and a range of preventative
measures and increased treatment options were introduced.1 At that time there were approximately 125,000 people
with Type 2 diabetes in New Zealand and it was projected that this number would increase to 180,000 in 2011.1 This
estimate has already been far exceeded with approximately 270,000 people in New Zealand (5 – 7% of the population) currently
diagnosed with diabetes.2
The Ministry of Health has labelled this the “Diabesity” epidemic, relating the significant rise in the number of people
with Type 2 diabetes to the rising rates of obesity. People of Māori, Pacific or Indo-Asian ethnicity are two to
three times more likely to have diabetes and this is a major contributor to increasing health inequalities.2
Interventions to reduce morbidity and mortality from diabetes focus on education, prevention and early detection of
diabetes and its complications.
“Get Checked” yearly to prevent diabetes complications
Part of the Ministry of Health’s “Diabetes 2000 Initiative” was the implementation of the annual, free “Get Checked”
programme. The goal of the programme is to increase intervention before more serious complications of diabetes develop.
The “Get Checked” annual health review includes:
- HbA1c level
- Blood pressure, lipid profile, height and weight
- Kidney function (microalbuminuria)
- Assessment of peripheral circulation and sensation of the feet
- Retinal check (at least every two years)
- Follow-up plan for care
Information for the annual review can be collected throughout the year, or alternatively as a more formal “one-off”
process.
An annual check for people with diabetes is also a PHO Performance Programme (PPP) indicator.
The PPP goal is for at least 80% of all people with diabetes enrolled in a practice to have an annual diabetes review.
“The Diabetic Foot” is a common complication of diabetes
Peripheral neuropathy and arterial disease are common complications of diabetes and are the main risk factors for the
development of ulcers, infection and ultimately lower extremity amputation.3,4
Neuropathy results in ulcer formation and other foot complications by decreasing pain sensation and perception of pressure.
This also causes muscle imbalance that leads to foot deformity and impaired microcirculation and integrity of the skin.5
A foot affected by neuropathy is described as warm, dry and numb,3 although sensory neuropathy can be very
painful. Pain, burning and tingling that is especially worse at night and relieved by getting up and walking, is highly
suggestive of diabetic peripheral neuropathy.
A foot affected by peripheral arterial disease is described as cold and without detectable pedal pulses. The patient
often experiences pain when walking or if severe, at rest. Once ulcers form, the capacity for them to heal is compromised
by diminished blood flow in the foot.5 Wounds can deteriorate rapidly and patients are at increased risk of
developing serious infection.3
These syndromes are collectively referred to as “The Diabetic Foot” and this is one of the most common complications
occurring among people with diabetes in New Zealand.3
Management of “The Diabetic Foot” in primary care focuses on:
- Regular (at least yearly) screening for foot problems in people with diabetes to prevent ulcer formation
- Prompt treatment and referral, if required, for any detected foot problems
- Education about preventing foot problems from occurring or worsening
Screening for diabetic foot complications
| Classifying risk of ulceration4 |
| Normal sensation, palpable pulses, no deformity |
Low current risk |
| Evidence of neuropathy, absence of pedal pulse(s) |
Increased risk |
| Evidence of neuropathy, absence of pedal pulse(s) and skin changes or deformity |
High risk |
Foot checks should begin immediately after a person has a confirmed diagnosis of type 2 diabetes and at least yearly
thereafter (as part of an annual diabetes review). If the patient has characteristics that increase their risk of foot
complications (see opposite) or once evidence of diabetic foot complications has been detected, feet should be checked
every three to six months.3 Some patients at very high risk of foot damage e.g. loss of feeling in the foot,
no detectable pedal pulses or active ulceration may be considered for review even more frequently, i.e. every one to three
months. These recommendations are summarised in Table 1.
| Table 1: Recommended frequency of examination for diabetic foot complications |
| Stage of progression |
Recommended frequency of foot check
|
| Confirmed diabetes |
As soon as possible after diagnosis, annually thereafter |
| First signs of foot problems identified or patient at high risk |
Every three to six months
|
Active ulceration and infection or very high risk
|
Regular assessment until active problems resolved, then every one to three months |
Patients who have the following characteristics are at high risk of developing foot complications:3,4
- Peripheral neuropathy
- Peripheral arterial disease
- Previous foot ulceration or amputation
- Structural foot deformity
- Plantar callus
- Older age (>70 years)
- Māori or Pacific ethnicity
- Longer duration of diabetes
- Smoking
- Other diabetic complications e.g. retinopathy
- Renal impairment
- Continual use of inappropriate footwear
- Living in a lower socioeconomic area
Performing a foot check3-5
- Examine the foot to identify deformity e.g. abnormal foot shape, clawed or hammer toes, ulceration,
skin abrasions, erythema, swelling and pressure points. Assess the skin status i.e. colour, thickness, dryness, cracking.
Check if the foot is fixed or flexible by asking the patient to stand and observe whether the toes straighten. Assess
how well the patient cares for their feet by checking for cleanliness and trimmed nails. Examine carefully between the
toes for tinea pedis. Check whether the patient can both reach and see their feet.
- Ask the patient if they experience numbness or pain, including what type of pain e.g. burning, tingling,
and at what times e.g. walking, resting, day-time, night-time. Ask about the normal temperature of the foot.
- Assess for neuropathy using a 10 g monofilament (see below). A vibration
test, using a 128 Hz tuning fork or a biothesiometer may also be performed. Absent touch pressure, pin prick or vibration
sensation (in a “stocking distribution”), absent ankle reflexes, altered temperature sensation and dry, scaly skin are
suggestive of neuropathy.
- Assess peripheral circulation with thorough palpation of pedal pulses (dorsalis pedis and posterior
tibial). If there are no palpable pulses, and if a Doppler machine is available, calculate ankle brachial index (see
below) or consider referral to a vascular specialist (see sidebar). Absent pulses, calf claudication, absence of hair
on the feet, altered temperature (a cold foot) and thin, bluish skin are suggestive of peripheral arterial disease.6 A
bounding, easily detected pulse in a warm, dry foot is suggestive of autonomic neuropathy, which causes abnormal arterio-venous
shunting.
Referral criteria for vascular review
Criteria for referral to a vascular surgeon for a patient with a diabetic foot complication includes the following:
- Foot lesion (ulcer, gangrene) or suggestion of rest pain with peripheral arterial disease
- Deteriorating ulcer with known peripheral arterial disease or absent pedal pulses
- Ankle Brachial Index <0.5 or absolute ankle pressure <50 mmHg
- New foot lesion with previously treated peripheral arterial disease
- Symptomatic intermittent claudication at <200 m
- Acute diabetic foot sepsis
- Osteomyelitis of forefoot or metatarsals
- Acute osteomyelitis
Calculating ankle brachial index7
Equipment: Blood pressure cuff and hand-held Doppler machine
- Take the blood pressure in the arm (brachial pressure)
- Take the blood pressure in the ankle using the Doppler machine (ankle pressure)
- Calculate ankle brachial index by dividing systolic ankle pressure by systolic brachial pressure e.g. ankle pressure
is 120 mmHg and brachial pressure is 132 mmgHg, ankle brachial index is 120/132 = 0.9
| Normal |
0.9 – 1.2 |
Risk of vascular foot ulcer is small |
| Definite vascular disease |
0.6 – 0.9 |
Risk of vascular ulcer moderate, depending on other risk factors |
| Severe vascular disease |
Less than 0.6 |
Risk of vascular foot ulcer very high |
Ankle brachial index may not be able to be reliably calculated in some people with diabetes as the arteries in the
ankles may be calcified.
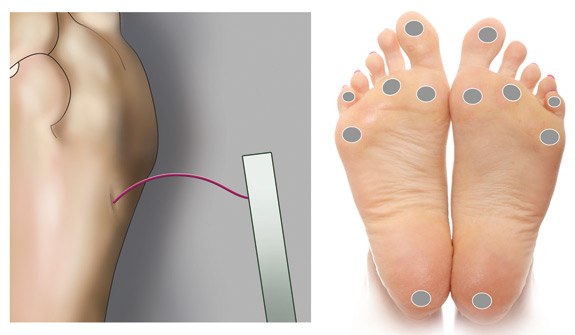
Performing a test using a monofilament3
A test using a 10 g monofilament is the recommended method for assessing for neuropathy of the foot. Loss of protective
sensation at any site on the foot indicates evidence of neuropathy, increasing the risk of ulceration and other complications.
Equipment: 10 g monofilament
Method:
- Place the patient in a supine position with shoes and socks removed
- Show the filament to the patient and bend it against their arm to illustrate that it is not painful
- Ask the patient to close their eyes and to say “yes” when they feel the filament on their feet. Do not prompt the
patient by asking “Did you feel that”?
- Place the filament on one of the designated sites on the foot (Figure 1), press it against the skin until the patient
indicates they can feel it, or a C shape is formed, and then lift it off. This should take approximately three seconds.
- Repeat this sequence at each of the designated sites on the feet and record findings
- Repeat again in the areas in which the patient did not indicate feeling the monofilament
- If evidence of neuropathy is detected, further assessment is required
Tips:
- Avoid tapping the filament against the skin or using rapid movements
- Choose the sites on the foot at random and try not to test sites in a predictable pattern that will allow the patient
to anticipate when and where the monofilament is likely to be positioned next
- Do not apply the filament directly on an ulcer, callous, scar or necrotic tissue. Apply the filament on near-by normal
tissue.
- The filament should be cleaned after use with an alcohol swab or dilute bleach solution and returned to its case
- Filaments should not be used for more than ten patients in 24 hours, as they may buckle
Figure 1: Monofilament bent to form a C shape. Recommended sites for cutaneous sensory
pressure perception testing using a monofilament.3
 Best Practice Tip: Regular callus removal should be performed in people with
diabetes and neuropathy. Calluses may hide underlying pressure ulcerations of the skin. It is recommended that patients
at risk of diabetic foot complications are referred to a podiatrist for removal of calluses.
Best Practice Tip: Regular callus removal should be performed in people with
diabetes and neuropathy. Calluses may hide underlying pressure ulcerations of the skin. It is recommended that patients
at risk of diabetic foot complications are referred to a podiatrist for removal of calluses.
Treating “The Diabetic Foot”
Lesions and ulcers detected during a foot check should be initially treated and any pain managed. It is recommended
that patients identified as being at increased risk of serious foot complications are then referred to a specialist multi-disciplinary
team for further management and care.3,4
Urgent referral to secondary care (within 24 hours) should be considered if:4
- An ulcer shows no signs of healing or becomes necrotic
- Significant swelling is present
- Discolouration of part or all of the foot is present
- There is suspicion of bone or joint involvement
Treatment of ulcers
Clean, debride and dress the wound
The wound may be cleaned, e.g. with saline, to remove surface bacteria and to allow assessment of swelling, redness
and discharge.
Surgical (using a scalpel or tissue nippers), mechanical (using saline and gauze) or hydrogel debridement (applying
a gel polymer dressing to the wound) can be used to remove non-viable or necrotic tissue, although this is not recommended
in the primary care setting when the debridement area is extensive. Surgical debridement is not recommended when sensation
to the foot is intact.8,9 There is limited evidence that hydrogel debridement increases the healing rate of
ulcers compared to gauze dressings or standard care.10 Hydrogel may also be preferable in the case of a painful
ulcer.9 Care must be taken to mask the edges of the wound, so surrounding tissue is not damaged.10
The ulcer should be kept clean and moist but free of excessive fluids.9 There is no evidence that one type
of dressing is superior to another for wound healing in diabetic foot ulcers. Dressings should be chosen based on their
comfort and durability when worn inside footwear, their ability to absorb exudate without plugging the wound and the ease
with which they can be regularly removed for checking the wound.4,9
If the wound does not appear to be infected, a long-term waterproof dressing can be applied and left in place for up
to one week before review. If the wound shows signs of infection, a non-adherent dressing can be applied and reviewed
every one to two days.
Off-load pressure from the foot
The central principle for healing any neuropathic ulcer is the reduction of pressure through pressure redistribution
(off-loading) until healing occurs. This involves resting the foot and using therapeutic footwear. If adherence to treatment
is problematic, some specialists may use a total contact cast to reduce pressure on the foot and allow more rapid healing.9
Graduated compression therapy (i.e. compression bandages or stockings) has an important role in healing and management
of venous leg ulcers and mixed aetiology venous ulceration, in people with diabetes and longstanding venous incompetence.
However, it does not usually have a role in healing neuropathic or arterial ulcerations associated with the diabetic foot
and may in fact worsen the condition. Specialist advice is recommended before considering the use of graduated compression
therapy in a person with diabetic foot complications.
Consider antibiotics
If the wound shows signs of infection e.g. erythema, oedema, foul odour or purulent discharge, antibiotic treatment
is indicated, either orally or intravenously (IV).
Consider admission to hospital for IV antibiotics for patients with extensive infection or where osteomyelitis is suspected
(see below).
When treating the infection in the general practice setting, a broad-spectrum antibiotic such as amoxicillin clavulanate
500/125 mg, three times per day, for five to ten days, may be used (as the infection is most likely to be polymicrobial).
Alternative agents are cefaclor or co-trimoxazole plus metronidazole. Swabbing the wound for microbiological analysis
is usually not necessary but can be helpful if the infection shows no sign of healing with the current antibiotic regimen.3,4
Osteomyelitis
Osteomyelitis is common in infected diabetic foot ulcers. Its presence greatly increases the risk of lower extremity
amputation. A probe can be inserted into the wound to check for bone involvement (a probe-to-bone test). A non-healing
ulcer, deep ulceration, extensive tissue loss, recurrent ulceration, previous osteomyelitis affecting the same bony region
or a history of discharge of bony fragments from an ulcer raises the likelihood of osteomyelitis being present. Visible
or palpable bone or joint structures make osteomyelitis a likely diagnosis. Referral to a multidisciplinary specialist
team is strongly recommended.
Monitor, review and consider referral
Regular review of the patient is encouraged. An infected wound should be reviewed and re-dressed every one to
two days. Note the size of the ulcer and whether it is decreasing.9
Check that the patient is following instructions for care and that they have removed pressure from the infected area.
If the ulcer shows no signs of healing or if infection is still apparent after antibiotic treatment, then referral to
a specialist team is strongly recommended.
Referral for vascular assessment is strongly recommended if limb ischaemia is present and compromising wound healing.
This can be corrected through revascularisation procedures.
Sub-optimal treatment can have serious long-term consequences for the patient. Referral to a specialist multidisciplinary
team for wound care and off-loading pressure can be considered with any diabetic foot complication to improve healing
times and patient outcomes.
Treatment of painful neuropathy
Pain associated with neuropathy is a common feature of diabetic foot complications. Neuropathic pain may be characterised
by altered pain sensation, numbness, burning or spontaneous pain.12
Treating neuropathic pain can be challenging and there is a lack of clear consensus as to which medicines to use and
in what order.12 Treatment should be tailored to individual circumstances and take into account factors such
as the severity of pain, coping strategies and lifestyle/occupational restrictions, e.g. a requirement to operate heavy
machinery would preclude using sedating medicines during the day.
Charcot’s osteoarthropathy
Charcot’s osteoarthropathy (or neuroarthropathy) is associated with severe peripheral neuropathy. It is a progressive
condition characterised by collapse and destruction of joints, fractures and bone destruction. In people with severe
diabetic neuropathy, Charcot’s osteoarthropathy most commonly manifests as acute swelling and deformity of the foot (without
open ulceration), leading to collapse of the pedal arch. This is a major risk factor for ulceration and subsequent amputation
of the foot.4,11
Acute Charcot’s osteoarthropathy can be confused with cellulitis, acute gout, osteomyelitis and abscess. In a patient
with a long duration of diabetes, a history of poor glycaemic control and peripheral neuropathy and no history of open
ulceration, Charcot’s osteoarthropathy should be considered first.11
People with suspected Charcot’s osteoarthropathy should be referred immediately for assessment and x-ray. Management
includes total contact casting and immobilisation of the joint. Bisphosphonate treatment is sometimes considered.4,11
After beginning any medicine (or medicine regimen) for treating neuropathic pain, the following aspects should be regularly
reviewed:12
- Pain control
- Adverse effects
- Mood
- Daily functioning
- Sleep patterns
Consider dose adjustment or adding or substituting another medicine if optimum control of these factors is not being
achieved.12
First-line pain management
Paracetamol may be trialled as first-line management for neuropathic pain and may be continued throughout any regimen.
Second-line pain management
If paracetamol alone is not adequate for controlling pain, a tricyclic antidepressant (TCA) may be added to the regimen
(or paracetamol substituted for a TCA).
Nortriptyline is the preferred TCA for neuropathic pain, due to fewer adverse effects than other TCAs. Initiate nortriptyline
at 10 mg per day (usually taken at night) and titrate dose upwards until pain is controlled. The dose should not usually
exceed 75 mg.12
Third-line pain management
If second-line pain management is insufficient, an anticonvulsant may be added to the treatment regimen, or the TCA
substituted for an anticonvulsant. Referral to, or discussion with, a pain specialist can be considered.
Carbamazepine and sodium valproate are both effective for neuropathic pain. Gabapentin has also traditionally been used
for neuropathic pain but recent evidence suggests that it has limited effectiveness for this indication.13
 For more information see “New
evidence shows less benefit of gabapentin for neuropathic pain” Snippets, BPJ 28 (June, 2010).
For more information see “New
evidence shows less benefit of gabapentin for neuropathic pain” Snippets, BPJ 28 (June, 2010).
Carbamazepine may be initiated at a dose of 100 mg per day. Increase the dose slowly until pain is controlled, to avoid
adverse effects such as nausea, vomiting and dizziness. Regular monitoring is required.
Opioids such as methadone or oxycodone may have a limited place in the treatment of neuropathic pain but their use is
not advised unless in consultation with a specialist in pain management.12
Adjuvants
Capsaicin cream and local anaesthetic gels may be trialled throughout a treatment regimen for neuropathic pain, They
should not be applied to broken/ulcerated skin.
 For more information about treating neuropathic pain, including considerations
for specific patient circumstances, see “Pharmacological management of neuropathic
pain”, BPJ 16 (Sept, 2008).
For more information about treating neuropathic pain, including considerations
for specific patient circumstances, see “Pharmacological management of neuropathic
pain”, BPJ 16 (Sept, 2008).
Considerations for Māori and Pacific people with diabetic foot complications
Māori and Pacific people with diabetes are at high risk of diabetic foot disease.
For Māori, tapu and noa are key concepts that underpin many practices. It is important to keep things that are
tapu (restricted) separate from things that are noa (unrestricted). In many cases these concepts or tikanga, align with
good health and safety practice.
 Best Practice Tip: Become familiar with the basic principles of tapu and noa,
and practical ways of respecting these concepts. For example:
Best Practice Tip: Become familiar with the basic principles of tapu and noa,
and practical ways of respecting these concepts. For example:
- For many Māori, it is inappropriate for their feet to be placed on a pillow, which is also used for the head.
Avoid propping feet up with a pillow during a foot examination or treatment.
- Māori may prefer their nail clippings and any other body parts (regardless of how minor it is perceived to be)
to be returned to them for disposal - ask them.
- Many Māori remove their footwear before entering their house or marae. Encourage the use of slippers or socks
to protect feet when inside, if outdoor shoes are considered unacceptable.
Preventing diabetic foot complications
The two main factors in preventing diabetic foot complications are:
- Maintaining optimal control of risk factors
- Educating about appropriate care of the feet
Optimal control of risk factors
The development of peripheral vascular disease and neuropathy, leading to foot complications, may be able to be avoided
or delayed with optimal management of diabetes and cardiovascular risk factors. This includes:
- Maintaining good glycaemic control – establish an individualised HbA1c target ( “HbA1c targets
in people with type 2 diabetes” BPJ 30, Aug 2010)
- Managing hypertension – New Zealand cardiovascular guidelines recommend reducing blood pressure to < 130/80 mm Hg
for people with diabetes, however this level may not be achievable for some people. In the presence of microalbuminuria
or renal disease more aggressive control may be required to reduce blood pressure to < 125/75 mm Hg.14
- Reducing blood lipid levels – aim for a reduction towards the target level of total cholesterol < 4.0 mmol/L,14 although
this level may not always be achievable ( “An update on statins” BPJ 30,
Aug 2010)
- Smoking cessation – provide advice and treatment options on how to quit
- Weight management – promote exercise and healthy diet
Educating about foot care
The three main aspects of foot care education have been identified as foot hygiene, awareness of fungal infections and
appropriate actions required for skin injuries.4 There is conflicting evidence on the effectiveness of educational
interventions on reducing the occurrence of foot ulceration, and which methods are best.15 Education is likely
to be effective in the short-term, but messages must be periodically reinforced for longer-term behavioural change.4
Providing advice about foot care
The following points can be discussed with patients in regards to the care of their feet:4
- Clean and thoroughly dry feet (including between the toes) every day
- Moisturise areas of dry skin and apply sun-screen if feet are exposed to the sun
- Inspect feet every day for abrasions, blisters, ulcers, redness, swelling or calluses
- Inspect between the toes for any signs of fungal infection
- Keep toenails trimmed, do not use “corn remover”, seek advice from a podiatrist about the treatment of corns or calluses
- Break in a new pair of shoes gradually, by first wearing for only an hour at a time
- Regularly inspect the inside of shoes for tears, sharp edges or foreign objects
- If neuropathy is present, extra vigilance is needed to avoid burns – check bath temperature, avoid hot water bottles,
electric blankets or foot spas
- Seek medical attention if any changes to the foot, abrasions or injuries are detected or pain or numbness develop
 Organisations such as Diabetes New Zealand have websites with downloadable patient
information and resources that can be helpful to reinforce advice: www.diabetes.org.nz
Organisations such as Diabetes New Zealand have websites with downloadable patient
information and resources that can be helpful to reinforce advice: www.diabetes.org.nz
Due to limited mobility or visual impairment, many people will be unable to adequately inspect and care for their feet.
Discuss methods to help self-examination such as the use of a mirror or the possibility of a family member or carer being
involved in regular foot care.
Selecting appropriate footwear
One of the most important aspects of preventing diabetic foot complications is wearing appropriate footwear. Patients
should be advised to always wear well-fitting, cushioned footwear (including slippers) to protect their feet from injuries.
Loose-fitting or open-toed footwear such as gumboots, jandals or sandals, and going barefoot should be avoided.
Patients (especially those at high risk) can be custom-fitted with specialised shoes and orthoses (insoles) by a podiatrist.
Specialised shoes for people with diabetes are usually made with extra depth and room to accommodate foot deformities
and orthoses. They have increased cushioning and reduce the pressure on certain parts of the foot, therefore reducing
the potential for ulcers to occur.16
Non-customised, specialised shoes are available “off-the-shelf” and are generally the same price as cushioned, high-quality
sports shoes, which are also an option. There is a lack of evidence of the superiority of custom-made therapeutic footwear
to off-the-shelf varieties in reducing the occurrence of ulcers.6 It appears that wearing a well-fitted, cushioned
pair of shoes, at all appropriate times, is more important than the actual type of shoe.
Orthoses (specially made insoles) can provide cushioning and redistribution of pressure loading. They may be worn in
specially designed or regular shoes.16
Socks and other hosiery should be well-fitted – neither too tight (leading to decreased circulation) nor too loose (leading
to rubbing and abrasions). Padded hosiery may protect the feet, reduce plantar pressure and reduce calluses.16 Socks
made from a breathable fabric such as cotton are preferable to those made from other fabrics.5
Referral criteria for podiatry services
Community diabetes specialist podiatrists hold contracts with their DHBs in most regions around New Zealand and undertake
primary care podiatry screening, assessment and treatment for the management of diabetic foot complications.
Secondary care hospital-based podiatrists are employed in most hospitals and can receive referrals for the acute management
of diabetes-related complications.
Contact your local DHB for details of funding for these services and referral criteria. In many areas, people with
diabetes related foot complications are able to access fully-funded podiatry services including supply of customised
therapeutic footwear and orthoses.
Ministry of Health criteria for podiatry referral for people with diabetes related foot complications17
At risk foot (criteria for referral to community-based podiatry
services)
|
High risk foot (criteria for referral to secondary care-based podiatry services) |
- Neuropathic ulceration
- A positive history of diabetic foot ulceration (and no current ulceration)
- Neuropathic foot with absence of protective sensation (patient cannot detect the 10 g monofilament at four or
more testing sites)
- Biothesiometer threshold >25 V
- Change to circulation and/or sensation with other risk factors present (see below)
- Neuropathy, musculoskeletal deformity and pre-ulcerative lesion
Risk factors:
| Long standing diabetes |
| Elevated HbA1c |
Nephropathy |
| Visual impairment |
Poor glycaemic control |
| Hypertension |
Smoking |
| Dyslipidaemia |
Obesity |
| Impaired mobility |
Social isolation |
| Perception of risk |
Male> 40 years |
|
- Past history of gangrene or amputation
- Peripheral vascular disease including:
- Absent pedal pulses and a history of claudication
- Ankle brachial index at 0.5–0.8 (indicating impaired arterial flow)
- Night pain
- Pre-ulcerated or ulcerated ischaemic lesion
URGENT referral to secondary care
- Neuropathic or neuro-ischaemic ulcers that have not demonstrated significant measurable improvement (30–40%) within
four weeks of treatment
- Ulcers presenting at > Grade 2 or indolent Grade 1 (graded by podiatrist)
- Cellulitis
- Systemic signs of infection
- Infection not responding to oral antibiotic therapy
- Radiological or clinical evidence of bone involvement including active Charcot’s neuroarthropathy
|
Acknowledgement
Thank you to Associate Professor Geoff Braadvedt, Physician and Endocrinologist, Department of Medicine,
University of Auckland and Angela Bayley, Diabetes Specialist Podiatrist, Orthotics Centre, Wellington for expert guidance
in developing this article.
References
- Ministry of Health. Diabetes Surveillance: Population-based estimates and projections for New Zealand, 2001-2011.
Public Health Intelligence Occasional Bulletin No. 46. Wellington: Ministry of Health, 2007. Available from:
www.moh.govt.nz (Accessed
Sept, 2010).
- Ministry of Health. A portrait of health: key results of the 2006/2007 New Zealand Health Survey. Wellington: Ministry
of Health, 2008. Available from: www.moh.govt.nz (Accessed Sept,
2010).
- New Zealand Guidelines Group (NZGG). Management of type 2 diabetes. Wellington: NZGG, 2003. Available from:
www.nzgg.org.nz (Accessed
Sept, 2010).
- McIntosh A, Peters J, Young R, et al. Prevention and management of foot problems in type 2 diabetes: Clinical guidelines
and evidence. University of Sheffield, Sheffield: National Institute for Clinical Excellence (NICE), 2003.
- McCulloch D. Evaluation of the diabetic foot: UpToDate, 2010. Available from:
www.uptodate.com (Accessed
Sept, 2010).
- Reiber G, Smith D, Wallace C, et al. Effect of therapeutic footwear on foot reulceration in patients with diabetes:
a randomised controlled trial. JAMA 2002;287(19):2552-8.
- Sorensen L, Wu M, Constantino M, Yue D. Diabetic foot disease: An interactive guide. Sydney: The University of Sydney.
Available from:
http://sydney.edu.au/medicine/diabetes/foot/Fexam1.html (Accessed
Sept, 2010).
- Dinh T, Veves A. Treatment of diabetic ulcers. Dermatol Ther 2006;19(6):348-55.
- McCulloch D, de Asla R. Management of diabetic foot lesions: UpToDate, 2010. Available from:
www.uptodate.com (Accessed
Sept, 2010).
- Edwards J, S S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev 2010;1:CD003556.
- Botek G. Charcot neuroarthropathy: an often overlooked complication of diabetes. Cleve Clin J Med 2010;77(9):593-9.
- National Institute for Clinical Excellence (NICE). Neuropathic pain: The pharmacological management of pain in adults
in non-specialist settings. NICE clinical guideline 96. London: NICE, 2010.
- Therapeutics Initiative. Gabapentin for pain: New evidence from hidden data. Therapeutics Letter 75. Vancouver: University
of British Columbia, 2009. Available from: www.ti.ubc.ca/letter75 (Accessed
Sept, 2010).
- New Zealand Guidelines Group (NZGG). New Zealand cardiovascular guidelines handbook. 2nd ed. Wellington: NZGG, 2009.
- Dorresteijn J, Kriegsman D, Valk G. Complex interventions for preventing diabetic foot ulceration. Cochrane Database
Syst Rev 2010;1:CD007610.
- Spencer S. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database Syst
Rev 2000;3:CD002302.
- Ministry of Health. Tier 3: Podiatry for people with at risk/high risk feet. Service specification. Wellington: Ministry
of Health, 2004. Available from: www.moh.govt.nz (Accessed Sept,
2010).