In this article 
View / Download
pdf version of this article
Key messages1
- Bisphosphonates decrease bone resorption by inhibiting osteoclasts and therefore have an important role in the
treatment of osteoporosis and prevention of corticosteroid-induced osteoporosis
- Risedronate has been fully subsidised, without restriction, on the Pharmaceutical Schedule since September, 2013.
Alendronate, etidronate and zoledronic acid are subsidised and indicated for the above
conditions. Alendronate (with or without cholecalciferol) and zoledronic acid require Special Authority approval for subsidy. Etidronate
does not require Special Authority approval but is considered to be less effective and requires a more complex
prescribing regimen compared to the once weekly dosing of alendronate and risedronate.
- There is increasing evidence that the majority of the benefit from bisphosphonate treatment occurs within the
first five years of treatment. The concept of a “drug holiday” applies to treatment with all bisphosphonates and is
thought to allow bone resorption to recover and aims to reduce the risk of rare adverse effects, e.g. osteonecrosis of
the jaw, in patients who are considered to be at a low risk of a fragility fracture.
- Risk factors for a fragility fracture include: previous fragility fracture, current use or frequent recent use of oral or
systemic corticosteroids, history of falls, family history of hip fracture, other causes of secondary osteoporosis,
low body mass index (less than 18.5 kg/m2 ), smoking, alcohol intake of more than 14 units per week for women and
more than 21 units per week for men.3 To calculate the ten year probability of a fracture, tools such as FRAX and
Garvan can be used.1
 For further information see:
Risedronate now fully subsidised: What is its place in practice? BPJ 56 (Nov, 2013)
For further information see:
Risedronate now fully subsidised: What is its place in practice? BPJ 56 (Nov, 2013)
New Zealand’s use of bisphosphonates – five year trend
Nationally between 2009/10 and 2010/11 the use of bisphosphonates increased by 5%, however, over the last four
years the number of patients taking a bisphosphonate has remained stable with approximately 55,000 people being
dispensed a prescription in any given year (Figure 1).
The use of risedronate, which has been fully subsidised since September 2013, and zoledronic
acid, a once-yearly
infusion, have rapidly increased since their introduction. These two medicines now account for 20% of bisphosphonate
use (Figure 1). When making the decision to initiate a patient on a bisphosphonate, risedronate is the first-line choice.
Zoledronic acid is considerably more expensive# ($600 per year) than either risedronate
($12 per year) or alendronate ($155 per year) and should be reserved for patients who are unable to tolerate an oral bisphosphonate.
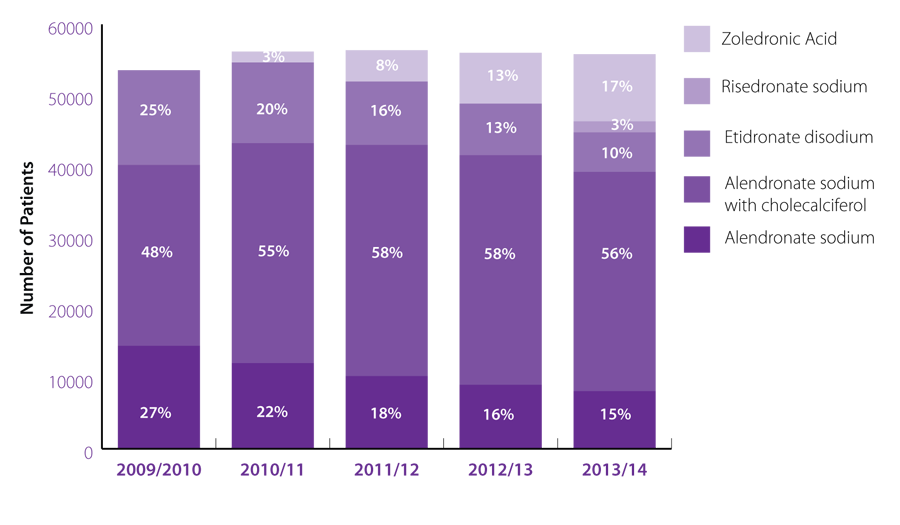
Figure 1. National bisphosphonate use July 2009 – June 2014
Sample Practice Data
Table 1 shows the number and proportion of patients registered to Sample Medical Centre
who received a bisphosphonate between Jul 2012–Jun 2013 and Jul 2013–Jun 2014
Table 1. Bisphosphonate use at sample practice 2012-2014
|
Number (%) of patients 2012/13 |
Number (%) of patients 2013/14 |
| Alendronate sodium |
12 (18%) |
10 (14%) |
| Alendronate sodium with cholecalciferol |
33 (49%) |
32 (45%) |
| Etidronate disodium |
12 (18%) |
10 (14%) |
| Risedronate sodium |
0 (0%) |
5 (7%) |
| Zoledronic acid |
11 (16%) |
14 (20%) |
| Total Patients* |
68 |
71 |
* Some patients may be counted twice if they have changed bisphosphonate medicines during the 12 month period
Drug Holiday
While the optimal duration of bisphosphonate treatment has yet to be established, it is now recommended that the continued use
of a bisphosphonate should be re-evaluated in individual patients at regular intervals based on the benefits and potential risks,
particularly after five or more years of use.
Nationally |
Sample Medical Centre |
48% |
of all patients dispensed a bisphosphonate (n=25,757) in 2013/14 |
|
53% |
of all patients dispensed a bisphosphonate (n= 34) in 2013/14 |
|
received a bisphosphonate dispensing every year for the last five years. These patients may benefit from a drug holiday if
they are considered to be at lower risk of fragility fractures.2,3 If treatment is stopped, ensure regular follow-up which may include a
falls risk assessment.
- Risedronate now fully subsidised: What is its place in practice? Best Practice Journal,
Issue 56 2013. Available at www.bpac.org.nz (Accessed Nov, 2014)
- Canadian Family Physician April 2014 vol. 60 no. 4 324-333
- Osteoporosis: assessing the risk of fragility fracture. National Institute for Health and Care excellence.
Available at www.nice.org.uk ( Accessed Nov, 2014)
# Prices do not include any rebates, markups or dispensing fees