Hyperglycaemia during pregnancy is associated with a range of adverse outcomes which can affect both mother and child, and can occur during pregnancy, childbirth or later in life. Due to physiological changes associated with pregnancy, women are at increased risk of developing diabetes, or having worsening glycaemic control if they have pre-existing diabetes. In December, 2014, the Ministry of Health released the Screening, Diagnosis and Management of Gestational Diabetes in New Zealand clinical practice guideline and some changes to testing for gestational diabetes are recommended. In this article, we summarise the recent Ministry of Health guidelines, with a focus on the role of the general practitioner in testing for undiagnosed diabetes early in pregnancy and monitoring for the development of type 2 diabetes after pregnancy.
 View / Download pdf version of this article
View / Download pdf version of this article
What is new?
- All pregnant women should be tested for undiagnosed diabetes using HbA1c prior to 20 weeks’ gestation
- Pregnant women with HbA1c ≥ 50 mmol/mol should be referred to a diabetes in pregnancy clinic
- Pregnant women with HbA1c 41 – 49 mmol/mol should be offered lifestyle advice to reduce risks of adverse
maternal and fetal outcomes; local protocols may recommend that these women are also referred to a diabetes
in pregnancy clinic
- At 24 to 28 weeks’ gestation, women are recommended to undergo an oral glucose tolerance testing regimen, which
is dependent on their initial HbA1c result
- HbA1c is used to monitor glycaemia postpartum in women who have had gestational diabetes, beginning
at three months after birth
Pregnancy is a time of significant metabolic change when a woman’s physiology adapts to meet the challenges of gestation.
Insulin sensitivity is decreased by as much as 50 to 60% during pregnancy, a level comparable to that seen in people with
type 2 diabetes or impaired glucose tolerance.1 This change in insulin sensitivity is thought to be caused
by endocrine signals from the growing placenta, and has evolved to aid fetal development.2 During pregnancy
the mother’s pancreas typically responds with beta-cell and islet hyperplasia to enable greater insulin production and
regulate blood glucose levels.1 Women who do not produce enough insulin to compensate for this transitory
increase in insulin resistance develop gestational diabetes. These women often have risk factors for the development of
type 2 diabetes and a higher level of insulin resistance before pregnancy.1 After childbirth, the insulin
resistance associated with pregnancy usually resolves, as does the need for treatment, if this has been required.
Maternal hyperglycaemia during pregnancy leads to fetal overgrowth (macrosomia), which is associated with an increased
risk of difficulties in delivery (shoulder dystocia, third or fourth degree perineal tears, postpartum haemorrhage)
and also a higher rate of caesarean section. Both maternal obesity and excessive gestational weight gain can
cause macrosomia, independently of gestational diabetes. The more serious complications, such as congenital malformation
and stillbirth, are largely confined to those women with previously unrecognised diabetes (usually type 2) that
has come to light as “gestational diabetes” (Table 1).
Pregnant women with intermediate glycaemia but without diabetes are also at risk of the same adverse outcomes
The Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study assessed the association of glucose tolerance with pregnancy
outcomes in over 25,000 pregnant women in nine countries who were below the diagnostic threshold for diabetes.3 Increasing
maternal glycaemia was associated with an increased risk of the infant being above the 90th centile of birth weight and
pre-eclampsia. There were modest associations with increased risks of neonatal hypoglycaemia, caesarean delivery, premature
delivery, shoulder dystocia or birth injury, and the infant requiring neonatal intensive care.3 This study
shows that increasing glycaemia, even if below the threshold for diagnosis of gestational diabetes, is potentially detrimental
to the fetus. The lowest risk pregnancy in terms of maternal glycaemia is one where the mother has blood glucose levels
as close to normoglycaemic as possible; in this study the lowest risk category was mothers with results on a 75 g oral
glucose tolerance test of fasting plasma glucose ≤ 4.2 mmol/L, one hour glucose levels ≤ 5.8 mmol/L or two hour glucose
levels ≤ 5.0 mmol/L.3
Which women are most at risk of diabetes during pregnancy?
Females with any of the following characteristics are at increased risk of undiagnosed diabetes or developing diabetes
during pregnancy:4
- A personal history of gestational diabetes or intermediate hyperglycaemia
- A previous infant > 4 kg at birth
- Increasing maternal age, particularly age over 35 years
- A first degree relative with diabetes
- A body mass index (BMI) ≥ 27 kg/m2 in an Indo-Asian person or ≥ 30 kg/m2 in other ethnicities
- Polycystic ovary syndrome
- Cardiovascular disease, hypertension, or elevated total cholesterol
- Physical inactivity
- Excessive gestational weight gain
- Long-term use of steroid (glucocorticoid) or antipsychotic medicines
- Acanthosis nigricans (hyperpigmentation of the skin)
The incidence of diabetes in pregnancy in New Zealand
The prevalence of diabetes has increased in New Zealand over recent decades and is currently around 5.8%; approximately
90% of whom are people with type 2 diabetes.4, 7 Data from the New Zealand Adult Nutrition Survey 2008/09
show that 1.5 – 1.8% of women aged between 25 and 44 years reported a diagnosis of diabetes with another 1.1 – 2.0% having
previously undiagnosed diabetes, highlighting that for every woman around childbearing age with diagnosed diabetes there
is another with undiagnosed diabetes.8 In New Zealand from 2001 to 2012 there was an annual increase of 13.9%
in the rate of gestational diabetes, with 4.9% of expectant mothers affected in 2012.4 It is not known how
many pregnancies in New Zealand are to mothers with pre-existing diabetes. Increases in the rates of gestational diabetes
and type 2 diabetes are likely to be due to changes in shared risk factors, such as physical inactivity and obesity.
There are marked differences in the rate of gestational diabetes between ethnicities in New Zealand: Asian (8.1%), Middle
Eastern, Latin American and African (7.5%), Pacific (7.2%), Māori (3.3%) and European (3.3%).4 However, it
has been suggested that the lower recorded rate among Māori may be due to lower rates of testing.4 Rates
appear to be increasing more rapidly in the Auckland and Northland regions.4
Table 1: Adverse outcomes for mothers with hyperglycaemia during pregnancy and their children4,5
Diabetes during pregnancy increases the risk of adverse outcomes for women:
Complications during pregnancy:
- Hypertension
- Polyhydramnios
- Pre-term labour
Complications during labour:
- Shoulder dystocia
- Operative vaginal delivery
- 3rd and 4th degree perineal tear
- Caesarean section
- Postpartum haemorrhage
In later life:
|
Diabetes during pregnancy increases the risk of adverse outcomes for infants:
Major complications with previously unrecognised diabetes:
- Stillbirth
- Congenital malformation
- Miscarriage
- Perinatal death
Fetal development complications:
- Macrosomia*
- Large for gestational age*
Birth traumas and complications during and after birth:
- Shoulder dystocia
- Bone fractures
- Brachial plexus palsy
- Hypoglycaemia
- Hyperbilirubinaemia
- Neonatal hypoglycaemia
|
Women with intermediate glycaemia have an increased risk of:
- Caesarean section
- Premature delivery
- Shoulder dystocia or birth injury
- Pre-eclampsia
|
Infants born to women with intermediate glycaemia have an increased risk of:
- Large for gestational age
- Neonatal hypoglycaemia
- Shoulder dystocia or birth injury
- Intensive neonatal care
- Hyperbilirubinaemia
|
Testing for glycaemia pre-conception
In women with known diabetes or those with a previous history of gestational diabetes, the ideal scenario is for pregnancies
to be planned and glycaemic control prior to pregnancy to be as optimal as possible.
Women with a history of gestational diabetes are at high risk of a having gestational diabetes during a
subsequent pregnancy. Rates of recurrence from 30% to 84% have been reported, with the highest rates in women who needed
insulin treatment during their previous pregnancy.4 For women with a previous history of gestational diabetes
who report that they wish to become pregnant, HbA1c levels should be checked and lifestyle modification encouraged
where appropriate so that their pregnancy begins with blood glucose levels as close to normoglycaemic as possible.
Women with established diabetes are at higher risk of adverse pregnancy outcomes such as congenital
malformation, miscarriage, stillbirth and perinatal death (Table 1).4, 5 Ideally, women
with diabetes should use contraception until blood glucose control is established and then attempt to conceive while maintaining
good blood glucose control.5 Folate
supplementation to reduce the risk of neural tube defects is recommended for all women who are trying to get
pregnant, from one month before to 12 weeks after conception. Women with diabetes are recommended to take 5 g of folic
acid daily (most other women can take 800 micrograms daily.9
 For further information on pre-conception care in general practice, see: “A
healthy start”, BPJ 67 (Apr, 2015)
For further information on pre-conception care in general practice, see: “A
healthy start”, BPJ 67 (Apr, 2015)
Early pregnancy: the role of primary care in testing for diabetes
Recommendations for testing and diagnosis of diabetes in pregnant women have been the subject of much debate (see: “A
lack of evidence hampers consensus on how to test for gestational diabetes”). Despite the lack of consensus on testing
during pregnancy to identify women with gestational diabetes, the role of testing early in pregnancy and postpartum in
women with previous gestational diabetes is much clearer:4, 5
- There is good evidence that hyperglycaemia in early pregnancy resulting from undiagnosed diabetes (usually type 2)
results in adverse pregnancy outcomes and that treatment of women with diabetes during pregnancy improves the health
of mother and child
- There is good evidence that women with a history of gestational diabetes are at increased risk of future type 2 diabetes
There is, therefore, a sound evidence base to test for undiagnosed diabetes in women who become pregnant, in order to
identify those who can benefit from intervention, and for women who have had gestational diabetes to be monitored postpartum
and be offered advice and support to reduce their future risk of type 2 diabetes.
Ministry of Health guidelines now recommend that all women should be tested for undiagnosed diabetes early in pregnancy
(prior to 20 weeks’ gestation) using HbA1c. A schedule of oral glucose tolerance tests (OGTT) between 24 to
28 weeks’ gestation to detect gestational diabetes is also recommended, with the specific testing regimen dependent on
the HbA1c test result from early pregnancy (Figure 1).4
Testing for undiagnosed diabetes in early pregnancy
Key practice points:
- It is now recommended that all pregnant women undergo testing for pre-existing diabetes
- Use HbA1c prior to 20 weeks’ gestation to detect pre-existing diabetes
- Women with an HbA1c ≥ 50 mmol/mol should be referred to a diabetes in pregnancy clinic (Figure
1).
- Women with an HbA1c between 41 and 49 mmol/mol should be encouraged to adopt lifestyle measures to reduce
their risk of adverse pregnancy outcomes; local DHB protocols may vary as to whether to refer these women to a diabetes
in pregnancy clinic
Testing for pre-existing diabetes can be performed using HbA1c. Physiological changes which occur during
pregnancy cause red blood cell turnover to increase and HbA1c levels decline, so HbA1c should be
performed prior to 20 weeks’ gestation to improve accuracy.4 Whenever testing for diabetes using HbA1c,
clinicians should keep in mind that some clinical conditions can affect HbA1c levels and give misleading results
(see: Factors affecting the reliability of HbA1c testing).
HbA1c test is most easily done as part of the first antenatal blood test screen. If a patient is seen in
general practice after they have enrolled with a lead maternity carer (LMC), check that the first antenatal screen, including
HbA1c, has been completed.
HbA1c testing in early pregnancy will identify women with probable pre-existing diabetes (HbA1c ≥50
mmol/mol) but also creates a new diagnostic entity – that is women with an HbA1c of 41 to 49 mmol/mol. The
HAPO study showed that mothers with elevated glycaemia below the threshold for diagnosing diabetes are at risk of some
adverse pregnancy outcomes so these women should be encouraged to adopt lifestyle measures to reduce their risk of developing
gestational diabetes and the adverse pregnancy outcomes associated with elevated glycaemia (see: “Lifestyle
approaches”).
A recently published opinion piece argues that women with an HbA1c ≥ 41 mmol/mol should be referred immediately
for management to a diabetes in pregnancy clinic (rather than just those with an HbA1c ≥ 50 mmol/mol).15 There
is as yet no evidence from randomised controlled trials, however, that earlier pharmacological intervention in these pregnancies
improves outcomes (see: “Research into gestational diabetes testing in New Zealand”).
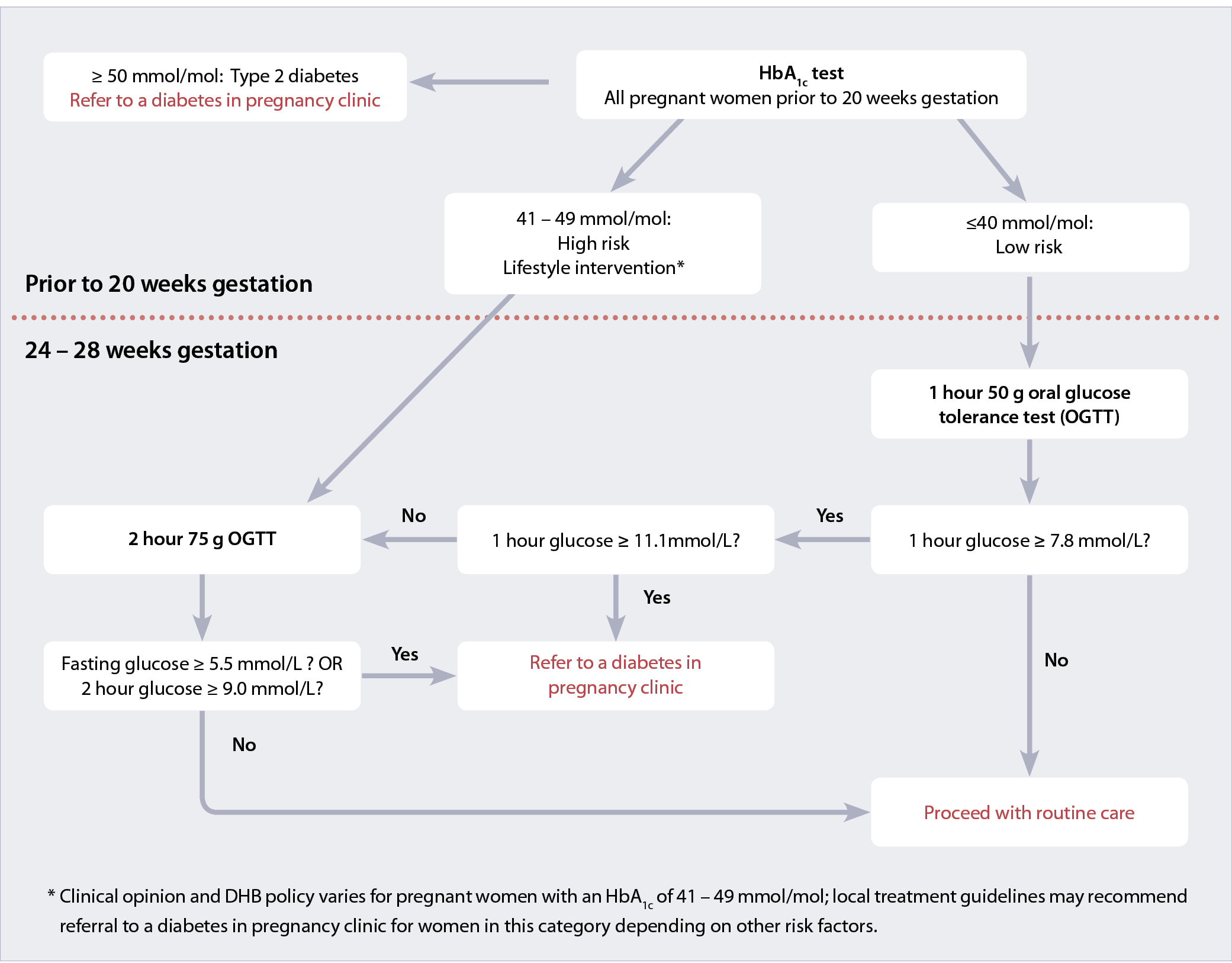
Figure 1: Screening and testing pathways for diagnosing diabetes in pregnancy4
Caring for patients with pre-existing diabetes who become pregnant
With rates of both type 1 and type 2 diabetes increasing in New Zealand, and maternal age at pregnancy increasing, it
is becoming more likely that clinicians will have under their care women with diabetes who become pregnant. In
addition to blood glucose monitoring with the aim of meeting recommended treatment targets (see: “What
are the treatment targets?”) these women usually require extra testing during pregnancy, in particular for retinopathy
and nephropathy.
Retinopathy testing
Diabetic retinopathy can progress during pregnancy.5 Women with diabetes who become pregnant should undergo
retinal photography during the first trimester, unless they have had this performed in the previous three months.5 Follow-up
ophthalmology examinations during pregnancy may be indicated depending on the degree of retinopathy.
Renal testing
Nephropathy during pregnancy is associated with an increased risk of pre-eclampsia, fetal growth restriction and pre-term
birth. Ideally, women with pre-existing diabetes should have renal function tests performed in the three months prior
to pregnancy, or if not, early in pregnancy at the first point of contact with the clinician.5 A protein:creatinine
level of 30 mg/mmol reflects a daily protein excretion of 300 mg and is the recommended test for the presence of proteinuria
in pregnancy.18 A serum creatinine level > 90 micromol/L accompanied by hypertension after 20 weeks’ gestation
is diagnostic of pre-eclampsia.18
 For further information on other routine laboratory testing during pregnancy, see:
www.bpac.org.nz/BT/2011/July/pregnancy.aspx
For further information on other routine laboratory testing during pregnancy, see:
www.bpac.org.nz/BT/2011/July/pregnancy.aspx

A lack of evidence hampers consensus on how to test for gestational diabetes
In general there is a low quality of evidence available to guide recommendations for testing for hyperglycaemia in pregnancy;
in particular, which screening strategies result in the best health outcomes for mother and child at the end of pregnancy
has not been thoroughly assessed.4 As a result, recommendations for how to test for hyperglycaemia during
pregnancy vary, principally for which type of oral glucose tolerance test to perform during pregnancy and what glucose
level cut-offs should be adopted to identify patients with gestational diabetes.4, 10 In New Zealand, testing
rates have been noted to vary across District Health Boards.11 A National Gestational Diabetes Mellitus Technical
Working Party published recommendations in 2008 to encourage alignment and standardisation across the country.11
The Maternity Quality Initiative Expert Working Group, formed in 2009 by the Ministry of Health, identified a need for
evidence-based guidelines for the diagnosis and management of hyperglycaemia in pregnancy in New Zealand.12 These
guidelines were developed by a multidisciplinary team and published by the Ministry of Health in December 2014. The recommendations
in this guideline differ from those published by other sources: for example, the Royal Australian and New Zealand College
of Obstetricians and Gynaecologists (July, 2014) and Australasian Diabetes in Pregnancy Society (November, 2014) recommendations.13,
14
Later in pregnancy: testing and management is organised by the LMC and diabetes in pregnancy clinic
Ministry of Health guidelines now recommend that oral glucose testing later in pregnancy (at 24 to 28 weeks) be tailored
to the patient’s early HbA1c results. This testing would usually be organised by the midwife. Most women will
be at low risk of developing gestational diabetes and can undergo a 50 g oral glucose challenge test. This test has a
good negative predictive value, so that women who test negative are at low risk of developing hyperglycaemia and the associated
risks of adverse pregnancy outcomes. Therefore, for most women the burden of testing is limited to a one hour test. Women
who test positive on the 50 g oral glucose challenge should undergo a 75 g two hour oral glucose tolerance test.
Women who are at increased risk of developing gestational diabetes (initial HbA1c results of 41 – 49 mmol/mol)
should proceed directly to a 75 g two hour oral glucose tolerance test without undergoing an initial 50 g oral glucose
challenge test (Figure 1).
There is some disagreement as to whether women with additional risk factors should proceed straight to a 75 g oral glucose
tolerance test even if their initial HbA1c screening result is ≤ 40 mmol/mol. For example, there is concern
that an obese woman may test negative on a 50 g glucose challenge as the glucose amounts are not adjusted for body weight,
potentially missing a diagnosis.15
Women who have pre-existing diabetes or have probable undiagnosed diabetes detected with initial HbA1c screening
should be under the care of a diabetes in pregnancy team. Further oral glucose tolerance testing is unlikely to be indicated.
One scenario which is not covered by the Ministry of Health guidelines is which testing procedure should be performed
for women who do not have an initial HbA1c result available. Given that an HbA1c test can be ordered
with the first antenatal blood tests it is likely that women without an HbA1c measurement in early pregnancy
also have a lower level of engagement with health services, and as a result may be at increased risk of adverse pregnancy
outcomes. A reasonable course of action would be to request that these women undergo a 75 g two hour oral glucose tolerance
test.
Women with diabetes during pregnancy may require oral hypoglycaemic medicines or insulin
Women who have difficulty reaching blood glucose targets or who have high initial blood glucose levels at diagnosis
are likely to require hypoglycaemic medicines, such as metformin or insulin injections (see: “What
are the treatment targets?”).
The regimen of medicines used to control the patient’s blood glucose levels will be determined by the diabetes in pregnancy
clinic, tailored to their treatment preferences and the degree of hyperglycaemia.5, 20 Metformin is the preferred
first-line treatment, as the risk of hypoglycaemia is lower than when using insulin and many patients prefer the ease
of taking a tablet rather than using injections. Glibenclamide is recommended as a possible second-line oral hypoglycaemic
medicine by the Ministry of Health and National Institute for Health and Care Excellence, where blood glucose control
is insufficient with metformin and the mother is unwilling or unable to use insulin, or if she experiences intolerance
to metformin.4, 5 Patients using only metformin have better outcomes than those using only glibenclamide.4,
5
Factors affecting the reliability of HbA1c testing
Measuring HbA1c is an indirect method of testing glycaemia; it relies on the glycation of haemoglobin over
the lifespan of an erythrocyte rather than directly measuring levels of glucose in the blood. Various clinical
conditions can affect erythropoiesis or erythrocyte destruction and influence haemoglobin levels or lifespan,
or can affect the chemical reactions which cause glycation of haemoglobin or used in assays to measure HbA1c.16 The
best workaround for clinicians when confronted with HbA1c results which do not appear to line up with a patient’s
presentation is to avoid the problems of indirect testing by simply ordering a blood glucose test; either
a fasting plasma glucose > 7 mmol/L or a random plasma glucose > 11.1 mmol/L can be used to diagnose diabetes.17
Patients with haemoglobinopathies may have altered HbA1c results depending on the type of assay used, with
the direction of change depending on the specific diagnosis. Other factors which may potentially give erroneous HbA1c results
include:16
Factors which can increase HbA1c :
- Alcohol intake
- Iron or vitamin B12 deficiency
- Hyperbilirubaemia
- Renal failure
- Opiate use
- Splenectomy
Factors which can decrease HbA1c :
- Erythropoietin, iron or vitamin B12 administration
- Ingestion of antioxidants such as vitamin C or E
- Very high triglyceride levels
- Chronic aspirin use
- Splenomegaly
- Rheumatoid arthritis
- Use of antiretrovirals
Women with gestational diabetes should self-monitor blood glucose
Self-monitoring and laboratory measurement of glucose levels during pregnancy are used as the key tests to guide treatment,
not HbA1c. While HbA1c is useful for monitoring long-term blood glucose control in non-pregnant
patients, it does not capture fluctuations in glucose concentrations, and evidence suggests that targeting treatment to
postprandial glucose levels results in the best outcomes for both mother and child in gestational diabetes. Furthermore,
physiological changes affect HbA1c levels during pregnancy, causing mean levels to drop compared with non-pregnant
women for the same degree of hyperglycaemia.4, 5
What are the treatment targets?
Recommended treatment targets for self-monitored (capillary fingerprick) blood glucose levels are:4, 20
- Pre-prandial (fasting): ≤ 5.0 mmol/L
- Post-prandial, either ≤ 7.8 mmol/L at one hour or ≤ 6.7 mmol/L at two hours
Ministry of Health guidelines recommend that women with gestational diabetes should aim to have > 90% of blood glucose
measurements in a week fall within the targets for glucose levels. If more than 10% of measurements fall outside of these
ranges, treatment should be reassessed.4
Research suggests that the closer to normal blood glucose is during pregnancy, the lower the risk of maternal and neonatal
complications. The treatment of all types of diabetes is a balancing act between attaining good glycaemic control while
minimising the risk of hypoglycaemia. Maintaining blood sugars that are too low can cause intrauterine growth restriction.5,
20
Lifestyle approaches are the cornerstone of reducing the risk and burden of diabetes in all people
A healthy diet and regular exercise are the cornerstones of preventing hyperglycaemia in all people, regardless of whether
they are pregnant. All women with diabetes during pregnancy should be offered specialist dietary advice.4 A
combined dietary and exercise approach is recommended which emphasises a balanced, healthy diet and encourages patients
to be active for at least 30 minutes a day most days of the week unless there are clinical contraindications to physical
activity. Limiting strenuous exercise may be necessary as pregnancy progresses and women should consult with their LMC
or diabetes in pregnancy team regarding appropriate physical activity.
A key goal is to limit gestational weight gain in those who are already overweight. The United States Institute of Medicine
released guidelines in 2009 for healthy ranges of weight gain during pregnancy depending on a woman’s pre-pregnancy body
mass index (BMI) (Table 2).19 Women who gain more than these amounts are at increased risk of developing
gestational diabetes, pregnancy-associated hypertension, complications during delivery and postnatal outcomes such as
subsequent weight retention after pregnancy and unsuccessful breastfeeding.19 Infants born to mothers with
excessive weight gain during pregnancy are at an increased risk of neonatal mortality, being large for gestational age,
and subsequent development of childhood obesity.19
Table 2: Recommended ranges of weight gain for pregnant women* 19
| Pre-pregnancy BMI (kg/m2) |
Rate of weight gain in 2nd and 3rd trimester (kg per week) |
Total weight gain (kg) |
| < 18.5 |
0.44 – 0.58 |
12.5 – 18 |
| 18.5 – 24.9 |
0.35 – 0.50 |
11.5 – 16 |
| 25.0 – 29.9 |
0.23 – 0.33 |
7 – 11.5 |
| ≥ 30 |
0.17 – 0.27 |
5 – 9 |
*N.B. These ranges are for singleton pregnancies. Larger increases in weight are acceptable for women with multiple fetuses.
After pregnancy: general practitioners should reassess glycaemic status
After pregnancy: general practitioners should reassess glycaemic status
Following a pregnancy affected by gestational diabetes, maternal hyperglycaemia may either resolve completely, or persist
– either as intermediate hyperglycaemia or as established diabetes.
Women with previous gestational diabetes have an approximately six to eight-fold higher risk of developing type 2 diabetes
than women who have been pregnant without diabetes, and may be at increased risk of developing type 1 diabetes.4,
5 Five year incidence rates of type 2 diabetes of 18% to 50% have been reported in women with a history of gestational
diabetes.21 The best approach is for preventive measures to begin as soon as the mother can manage.
HbA1c testing at three months postpartum or later is recommended
Clinicians should aim to assess the glycaemic status of all women who have had gestational diabetes. Research suggests
that many women with a history of gestational diabetes in New Zealand are not subsequently tested.
Oral glucose tolerance testing at six weeks after birth has been, until recently, the recommended way to assess glycaemic
status following gestational diabetes, and this is still recommended in many overseas’ guidelines. However, the Ministry
of Health guidelines now recommend using HbA1c at three months after birth, with the possible addition of
fasting blood glucose. It is hoped that this change will facilitate greater uptake of testing given that many patients
find the oral glucose tolerance test inconvenient. This change also means that three month postnatal testing uses the
same test as later annual monitoring, so that clinicians are better able to determine if glycaemic control has deteriorated.4
Annual testing thereafter using HbA1c is recommended (Table 3). Opportunistic or scheduled patient appointments,
such as seeing the clinician when they bring their infant for vaccinations, can be a good time to test the mother for
diabetes, or clinicians can set up an electronic reminder to ensure HbA1c testing is performed at appropriate
intervals. Patient reminders such as a letter, email or text are likely to improve rates of postpartum testing for diabetes.22
Testing HbA1c at three months postpartum has low sensitivity but high specificity for detecting type 2
diabetes in women who have had gestational diabetes compared to a 75 g oral glucose tolerance test. It is likely to detect
those with the highest levels of glycaemia and most in need of treatment. Fasting blood glucose testing can also be performed
at three months postpartum at the clinician’s discretion, which increases the sensitivity of three month testing for detecting
patients with type 2 diabetes.4
Table 3: Tests and appropriate follow-up at three months postpartum for women who have had gestational diabetes4
| HbA1c level (mmol/mol) at three months |
Diagnosis or risk category |
Patient care and testing |
≥ 50 and with symptoms of diabetes |
Diabetes* |
Proceed to treat and follow-up as per diabetes guidelines |
≥ 50 and asymptomatic |
Possible diabetes* |
Repeat testing with HbA1c or fasting blood glucose |
41 – 49 |
High risk of diabetes* |
Provide diabetes prevention advice regarding diet and exercise measures. Metformin treatment may be considered.
(see: “Patient and clinician actions that can prevent future diabetes”)
Re-test HbA1c in six months and then annually thereafter |
≤ 40 |
Medium risk of diabetes* |
Annual HbA1c testing |
* In most cases this will be type 2 diabetes; occasionally patients with early stage type 1 diabetes
or rarely monogenic diabetes (caused by single gene mutations) may be encountered.
Patient and clinician actions that can prevent future diabetes
Lifestyle measures are the cornerstone of preventing and treating type 2 diabetes and clinicians should encourage women
with a previous history of gestational diabetes to adopt a diet and exercise regimen which can reduce their risk of future
diabetes. In addition to lifestyle measures, other advice and treatments which clinicians can offer include:
Breastfeeding: Mothers who breastfeed usually lose more weight than mothers who do not, and various
cohort and observational studies have found that mothers who have breastfed have reduced risks for a number of metabolic
diseases across their lifetime, including obesity, diabetes, hypertension, hyperlipidaemia and cardiovascular disease.23
Metformin: Women with an HbA1c of 41 – 49 mmol/mol who have not been successful in reducing
their level of glycaemia with lifestyle measures could be offered metformin.4 Data from the United States
Diabetes Prevention Program study suggests a number needed to treat (NNT) of seven patients for ten years to
prevent one case of type 2 diabetes.24 Clinicians may wish to consult an endocrinologist or diabetes specialist
when considering this treatment approach.
Research into gestational diabetes testing in New Zealand
The gestational diabetes mellitus study of detection thresholds (GEMS) study
Clinicians in any area of New Zealand may consider referring their pregnant patients to the GEMS study, currently being
conducted by the Liggins Institute at the University of Auckland. This study aims to randomise pregnant females to undergo
testing and treatment according to the New Zealand criteria for the diagnosis of gestational diabetes or the International
Association of Diabetes and Pregnancy Study Group (IADPSG) criteria in order gather better evidence regarding which criteria
produce the best outcomes for mother and child. The IADPSG recommend diagnosing gestational diabetes at lower cut-offs
of oral glucose tolerance test results than the New Zealand guidelines.
 For further information, see: www.ligginstrials.org/GEMS or
email: gems@auckland.ac.nz
For further information, see: www.ligginstrials.org/GEMS or
email: gems@auckland.ac.nz

The “Pre-diabetes in pregnancy: can early intervention improve outcomes” (PINTO) trial
The aim of the PINTO trial is to examine whether blood glucose monitoring and initiating treatment for hyperglycaemia
in women with HbA1c levels between 41 – 49 mmol/mol in early pregnancy, can improve health outcomes compared
with lifestyle advice and follow up gestational diabetes screening at 24 – 28 weeks’ gestation. The first phase of the
trial is a feasibility study which will inform the main randomised controlled trial. Researchers will be recruiting women
in the National Woman’s Hospital (Auckland) or Christchurch Women’s Hospital catchment areas from 1 October, 2015. General
practitioners in these areas should refer women with an HbA1c ≥ 41mmol/mol directly to their local diabetes
in pregnancy clinic as soon as possible, where dietary and weight gain advice, triage, and consent will take place. A
mail out to general practitioners will take place prior to this date, to provide further information about the study.
It is hoped that this study will show that early intervention in this patient group, including blood glucose monitoring
and optimisation of blood glucose levels through dietary measures and medicines as required, will reduce pre-eclampsia,
neonatal morbidity and mortality without causing harm. It is also hoped that the study will reduce inequalities in health-related
outcomes for Māori and Pacific women, who have high rates of pre-diabetes and are the least likely to take up conventional
screening for gestational diabetes.
 For further information about PINTO,
contact Dr Ruth Hughes: ruth.hughes@cdhb.health.nz
For further information about PINTO,
contact Dr Ruth Hughes: ruth.hughes@cdhb.health.nz
Acknowledgement
Thank you to Professor Tim Cundy, Endocrinologist, Auckland DHB and Professor of Medicine,
University of Auckland and Dr Cam Kyle, Clinical Biochemist, Auckland for expert review of this article.
References
- Lacroix M, Kina E, Hivert M-F. Maternal/fetal determinants of insulin resistance in women during pregnancy and in
offspring over life. Curr Diab Rep 2013;13:238–44.
- Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sc 2015;370:20140066.
- Metzger BE, Lowe LP, et al with HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002.
- Ministry of Health (MoH). Screening, diagnosis and management of gestational diabetes in New Zealand. MoH, 2014.
Available from: www.health.govt.nz (Accessed Jul, 2015).
- National Institute for Health and Care Excellence (NICE). Diabetes in pregnancy: management of diabetes and its complications
from preconception to the postnatal period. NICE, 2015. Available from:
www.nice.org.uk/guidance/ng3 (Accessed Jul, 2015).
- Poolsup N, Suksomboon N, Amin M. Effect of treatment of gestational diabetes mellitus: a systematic review and meta-analysis. PLoS ONE 2014;9:e92485.
- Ministry of Health (MoH). Virtual Diabetes Register (VDR). MoH, 2015. Available from:
www.health.govt.nz (Accessed Jul, 2015).
- Coppell KJ, Mann JI, Williams SM, et al. Prevalence of diagnosed and undiagnosed diabetes and prediabetes in New
Zealand: findings from the 2008/09 Adult Nutrition Survey. N Z Med J 2013;126:23–42.
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Vitamin and mineral supplementation
and pregnancy. RANZCOG, 2014. Available from:
https://www.ranzcog.edu.au/college-statements-guidelines.html (Accessed Jul, 2015).
- Buckley BS, Harreiter J, Damm P, et al., on behalf of the DALI Core Investigator Group. Gestational diabetes mellitus
in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med 2012;29:844–54.
- Simmons D, Rowan J, Reid R, et al., National GDM Working Party. Screening, diagnosis and services for women with
gestational diabetes mellitus (GDM) in New Zealand: a technical report from the National GDM Technical Working Party. N Z Med J 2008;121:74–86.
- Ministry of Health (MoH). National maternity clinical guidance. MoH, 2014. Available from:
www.health.govt.nz/our-work/life-stages/maternity-services/national-maternity-clinical-guidance (Accessed Jul, 2015).
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Diagnosis of gestational
diabetes mellitus (GDM) and diabetes mellitus in pregnancy. Victoria, Australia: RANZCOG, 2014. Available from:
www.ranzcog.edu.au/doc/diagnosis-of-gestational-diabetes-mellitus-gdm-c-obs-07.html (Accessed Jul, 2015).
- Nankervis A, McIntyre H, Moses R, et al. ADIPS consensus guidelines for the testing and diagnosis of hyperglycaemia
in pregnancy in Australia and New Zealand. Australasian Diabetes in Pregnancy Society, 2014. Available from:
www.ranzcog.edu.au/doc/adips-gdm-guidelines.html (Accessed Jul, 2015).
- Rowan J, Allen H, Budden A, et al. New Zealand National GDM Guidelines: an alternative view of some good practice
points. Aust N Z J Obstet Gynaecol 2015;55:17–20.
- World Health Organisation (WHO). Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Geneva:
WHO, 2011. Available from:
www.who.int/diabetes/publications/diagnosis_diabetes2011/en/ (Accessed Jul, 2015).
- New Zealand Guidelines Group (NZGG). New Zealand Primary Care Handbook 2012. Wellington: NZGG, 2012. Available from:
www.health.govt.nz/publication/new-zealand-primary-care-handbook-2012 (Accessed Jul, 2015).
- SOMANZ: Society of Obstetric Medicine of Australia and New Zealand. Guideline for the management of hypertensive
disorders of pregnancy. Sydney, NSW: SOMANZ, 2014. Available from:
http://somanz.org/downloads/HTguidelineupdatedJune2015.pdf (Accessed Jul, 2015).
- Institute of Medicine, National Research Council of the National Academies. Weight gain during pregnancy: re-examining
the guidelines. Washington, DC: The National Academies Press, 2009. Available from:
http://iom.nationalacademies.org/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx (Accessed Jul, 2015).
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2015;38:S1–93.
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. A systematic review. Diabetes
Care 2002;25:1862–8.
- Middleton P, Crowther CA. Reminder systems for women with previous gestational diabetes mellitus to increase uptake
of testing for type 2 diabetes or impaired glucose tolerance. Cochrane Database Syst Rev 2014;3:CD009578.
- Mohammad MA, Haymond MW. The magic of mother’s milk. Diabetes 2012;61:3076–7.
- Aroda VR, Christophi CA, Edelstein SL, et al., Diabetes Prevention Program Research Group. The effect of lifestyle
intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the
Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100:1646–53.