Haematuria on dipstick
Haematuria can be classified as visible, also known as macroscopic or gross haematuria, or non-visible, also known
as microscopic haematuria.1 Haematuria can originate from numerous sites including the kidney, ureter, bladder,
prostate, urethra or other structures within the urogenital tract.
Urine dipsticks are a rapid and relatively sensitive (>80%) method for detecting haematuria in a freshly voided
sample of urine.2 However, as well as intact red blood cells (RBC), urine dipstick may also detect haemoglobin
from lysed RBC caused by haemolytic conditions, or myoglobin from crush injuries, rhabdomyolysis or myositis. As a consequence,
reports of specificity range from 65 – 99%.3 Significant haematuria occurs at readings of 1+ or above, and
trace levels should be considered negative.1
Urine microscopy is not routinely required for confirming a dipstick diagnosis of haematuria.1 However,
in some situations, after clinical evaluation, urine microscopy may be useful in helping to distinguish haematuria from
haemoglobinuria and myoglobinuria and to detect dysmorphic red blood cells and urinary casts indicating a medical renal
cause.
Visible haematuria (macroscopic)
Visible haematuria is primarily associated with urological conditions. Rarely, similar changes in urine colouration
may be due to other causes such as haemoglobinuria, myoglobinuria, beeturia (after eating beetroot), porphyria or medicines,
e.g. rifampicin and chlorpromazine.1 Haemoglobinuria can occur with haemolytic anaemia, which may be accompanied
by rapidly developing pallor, splenomegaly and jaundice due to an increased concentration of bilirubin. Myoglobinuria
is usually associated with rhabdomyolysis.
Non-visible haematuria (microscopic)
Transient, non-visible haematuria is common and, depending on the studied population, may be reported in as many as
39% of people.3 It is associated with a mixture of urological and glomerular causes. Persistent, non-visible
haematuria is defined as urine positive on two out of three consecutive dipsticks, e.g. over a one to two week period.
It is estimated to occur in 2.5 – 4.3% of adults seen in primary care.3
Assessing haematuria
Haematuria can be symptomatic or asymptomatic. Relevant lower urinary tract symptoms include dysuria, frequency, urgency
and hesitancy. Table 1 provides guidance when considering causes for haematuria. Anticoagulant
and anti-platelet medicines are more likely to exacerbate, rather than cause, haematuria. Therefore patients who are
taking these medicines who present with haematuria require investigation.1
Clinical suspicion of significant urological disease should be raised in people with haematuria with the following
risk factors:4
- History of recurrent visible haematuria
- Age over 40 years
- Current smoker or recent history of smoking
- History of recurrent urinary tract infection (UTI) or other urological disorders
- Occupational exposure to chemicals or dyes
- Previous pelvic irradiation
- History of excessive analgesic use
- Treatment with cyclophosphamide
Risk factors specific for bladder cancer include; family history, smoking, male gender and occupational exposure to
carcinogens, e.g. benzenes, organic solvents or aromatic amines.5
A clinical history and examination may indicate a possible source of bleeding. As urinary tract infection (UTI) is
a common cause of haematuria, this should first be considered and excluded. Non-visible haematuria is often transient
so persistence should be confirmed by the presence of two out of three positive dipstick tests, seven days apart.6
Investigating visible haematuria
If UTI or other obvious causes have been excluded, imaging of the urinary tract is indicated (see “Urinary
tract imaging – informed decision making” and Figure 1). Assessment by an Urologist and cystoscopy
will also be required in the majority of cases, although in young people (age less than 40 years with no risk factors
for urothelial malignancy) cancer is unlikely to be the cause. If investigations are normal, i.e. do not suggest a urological
cause, a nephrology opinion is required to exclude a medical renal cause, with urgency dependent on the continuing level
of haematuria.
Investigating non-visible haematuria with urinary tract symptoms
Non-visible haematuria is regarded as significant once transient causes, e.g. urinary tract infection (UTI) or exercise,
or benign causes, e.g. menstruation, have been excluded. Urinary tract imaging is indicated for all patients of any age
with recurrent, symptomatic, non-visible haematuria (see “Urinary tract imaging – informed decision
making” and Figure 1).1, 6, 11 Urological assessment and cystoscopy is also required
for patients aged over 40 years, or for patients with risk factors for urothelial malignancy.1 When lower
urinary tract symptoms are present in males aged over 40 years, digital rectal examination of the prostate and PSA testing
should be undertaken. Incidental, non-visible haematuria may be present when prostatic cancer is diagnosed, usually as
a result of associated benign prostatic hypertrophy. Typically, prostate cancer does not cause haematuria unless it is
at an advanced stage.12
Baseline assessment of blood pressure and renal function with testing of creatinine (eGFR), ACR / PCR and urine microscopy
for urinary casts and dysmorphic red cells are also recommended to identify patients with a renal medical cause for non-visible
haematuria.1, 6
Investigating asymptomatic non-visible haematuria
Age over, or under 40 years is used to determine the likelihood of there being a urological or renal medical explanation
for asymptomatic non-visible haematuria.1 For patients at higher risk of a urological cause, e.g. age over
40 or younger with risk factors for urothelial malignancy, urinary tract imaging is indicated (see “Urinary
tract imaging – informed decision making“). For those at low risk of a urological cause, renal ultrasound is indicated
and a nephrology opinion is recommended under any of the following circumstances:11
- eGFR < 30 mL/min/1.73m2 – Stage 4 or 5 CKD
- eGFR declining by > 5 mL/min in the previous year or > 10mls/min over the last five years
- Significant proteinuria ACR ≥ 30 mg/mmol or PCR ≥ 50 mg/mmol (proteinuria ≥ 0.5 g/24 hours)
- Uncontrolled hypertension ≥ 140/90 mmHg
- Unexplained visible haematuria following urological assessment where no cause was found
Table 1: Causes of haematuria that may be considered when assessing a positive dipstick7
| Common in primary care |
Transient/other |
Do not miss |
Consider |
- Urinary tract infection
- Urinary tract or kidney stones
- Prostatitis
|
- Menstruation
- Exercise-induced
- Benign prostatic hyperplasia
- Mild trauma
- Pseudohaematuria, e.g. beeturia
|
- Urinary tract, kidney or prostate malignancy
- Cardiovascular:
- - Kidney infarction
- - Kidney vein thrombosis
- - Prostatic varices
- Acute glomerulo-nephritis
- Severe infection:
- - Infective endocarditis
- - Kidney tuberculosis
- Papillary necrosis
- IgA nephropathy
|
- Urethral prolapse
- Foreign body
- Radiation cystitis
- Familial:
- - Thin basement membrane disease
- - Adult polycystic kidney disease
|
Primary care monitoring of unexplained haematuria
Primary care surveillance of unexplained haematuria requires annual assessment of urine dipstick, serum creatinine
(eGFR) and urine albumin:creatinine ratio (ACR), or urine protein:creatinine ratio (PCR). This should be conducted until
two consecutive negative urinalyses occur.13 Patients with stable chronic kidney disease (CKD) should be
monitored according to their stage of CKD. Patients should be referred back to urology if haematuria persists, or urinary
tract symptoms develop or increase.
 For further information see: “Making
a difference in chronic kidney disease” BPJ 22 (Jul, 2009).
For further information see: “Making
a difference in chronic kidney disease” BPJ 22 (Jul, 2009).
Urinary tract imaging – informed decision making
A computed tomography urogram (CTU) is regarded as the current gold standard for imaging in the investigation of visible
and non-visible haematuria. However, some regions have reduced access to CTU and funding constraints mean that intravenous
urogram (IVU/IVP) and ultrasonography still have a role when investigating patients at lowest risk of renal tract malignancy.
A CT should be performed in at least three phases; a non-contrast phase to detect urinary stones, a contrast phase
to evaluate structural, vascular, or infectious abnormalities of the renal parenchyma, and a delayed excretory phase
to outline the collecting system. This is often referred to as a triphasic-CT , CTU/IVP or CT-Haematuria.
The non-contrast phase of CT can detect renal stones with sensitivity of 94% to 98%, compared with 52% to 59% for IVU.15
CT is superior to ultrasound and IVU for detecting renal masses.16
CTU is the most comprehensive radiological method for evaluating the urinary tract for urolithiasis, renal masses,
and urothelial neoplasms in a single examination.13 Cystoscopy is still required to exclude a cause for haematuria
located in the bladder.
Women who are pregnant, or people who have a suspected allergy to the contrast media, may not be suitable for CTU imaging.
Pre-existing renal dysfunction may also be a contraindication for CTU.
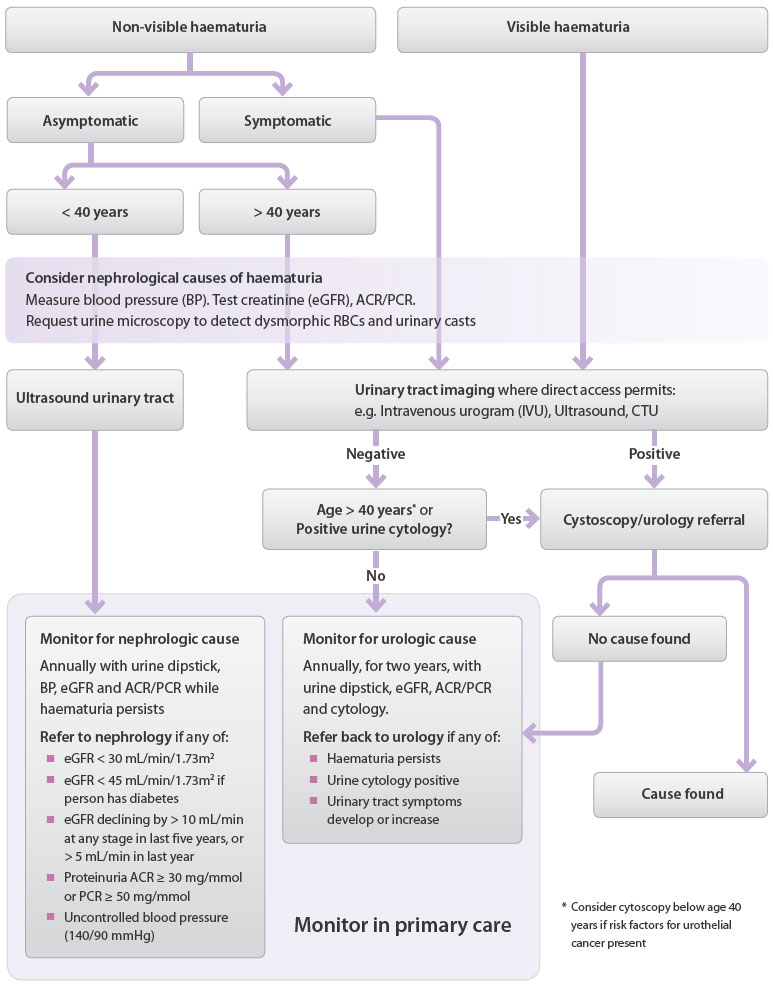
Figure 1: Investigation and referral algorithm for significant haematuria in adults once
UTI and benign causes have been excluded 1,6, 13, 14
Suspected UTIs and cancer risk in males
Urinary tract cancer (kidney and bladder) has a higher incidence in males than females. In New Zealand, in 2009, there
were 581 urinary tract cancer registrations for males, compared to approximately 300 for females.8 Treatment
is often curative if there is an early diagnosis when the malignancy is localised to the kidney and the immediately
adjacent tissue. Renal cancer is rare in people aged under 35 years, and bladder cancer is rare below age 50 years.9 Visible
haematuria is a common symptom of urinary tract cancer.
When examining males with a suspected UTI, consider the possibility of malignancy, especially in patients with risk
factors for cancer. Urine culture is recommended in all males with suspected UTI (in contrast to guidance for females
with uncomplicated UTI) to confirm a diagnosis and guide treatment.10 Males with a UTI that does not respond
to antibiotic treatment, or who have persistent haematuria, should be referred to an Urologist.10
Factors that increase the risk of UTI in males include:10
- Age > 65 years
- Institutional care
- Bladder outlet obstruction
- Previous urinary tract surgery or recent procedures, e.g. prostate biopsy
- Anal intercourse
- Immunodeficiency
Urine cytology should not be routinely used in the initial investigation of haematuria
Urine cytology is a non-invasive method of testing for bladder cancer, however it is not a “rule-out” test due to
low sensitivity of 40–76%.18 The test detects cancerous cells shed from any part of the urothelium. Reports
of specificity are as high as 98%.18 The sensitivity of urine cytology for detecting cancer is influenced
by the type of tumour present. Large, or high-grade tumours, or carcinoma in situ are more likely to shed cells and
the sensitivity for detecting these is high, however, sensitivity for low-grade cancer is reported to be 11%.18 As
60% of urothelial tumours are reported to present as low-grade and early-stage lesions, this has important implications
for the use of urine cytology as a detection tool for bladder cancer.18 Urine cytology results are also
dependent on operator skill and it is important to have an experienced pathologist interpret the results.17
Cystoscopy is the preferred technique for excluding bladder cancer as the cause of haematuria as it is reported to
have a specificity for malignancy of over 90% and the added advantage of being able to detect stones, vascular abnormalities
and infectious lesions, which can also cause haematuria.18 Furthermore, a study where 182 patients underwent
405 cytologies found that no patients with a positive cytology had a negative cystoscopic/radiological evaluation.17 This
suggests that the addition of cytology to an investigation of haematuria is unlikely to significantly increase the rate
of cancer detection when all high-risk patients proceed to cystoscopy and radiology. The role of urine cytology as an
investigation of haematuria is therefore being increasingly questioned.18 It should not be used as part
of a routine evaluation.12 It is acknowledged, however, that some regional guidelines include urine cytology
to aid triaging for cystoscopy. There may still be a role for cytology in circumventing the need for cystoscopy in high-risk
patients likely to require surgery, or as a monitoring method for patients with an undiagnosed cause of haematuria and
patients with a history of bladder cancer.18
Proteinuria on dipstick
People with normal kidney function excrete less than 150 mg of protein per day in their urine, approximately 20 mg of
which is albumin.24 Persistent protein excretion significantly above this level is a marker for kidney disease,
and kidney disease progression, and indicates an increased risk for cardiovascular events.25
Urine dipstick is a highly specific (97 – 100%) method for detecting proteinuria, however, the sensitivity of the test
for detecting low-end, but clinically significant proteinuria is reported to be 32 – 46%.26 Therefore in people
diagnosed with, or suspected of having diabetes, a more sensitive technique, i.e. albumin:creatinine ratio (ACR), is recommended
to quantify proteinuria.25
Proteinuria on dipstick in primary care is frequently an incidental finding and is often benign and transient.26 However,
the presence of proteinuria can also suggest endothelial/glomerular injury. The first step in assessment should be to
consider the possibility of a false positive result, which can be caused by alkaline urine (pH >7), gross haematuria,
mucus, semen or leukocytes.26
Confirm persistent proteinuria
Proteinuria may be transient or persistent. Transient, mild proteinuria can be caused by recent strenuous exercise,
standing for long periods (orthostatic proteinuria), pregnancy, UTI and acute febrile illness.26 Congestive
heart failure is a more serious cause of proteinuria that can also be transient. Orthostatic proteinuria is typically
absent in the morning, occurs in the afternoon and is seen mainly in young adults.26
Transient proteinuria can be confirmed by a repeat dipstick result which is negative, in the absence of any suspected
transient cause. Persistent proteinuria can be confirmed by two or more consecutive positive dipsticks over a one to two
week period.27
If persistent proteinuria on dipstick is present an ACR or PCR should be performed to quantify the level of proteinuria
(Figure 2). ACR is the preferred method for quantifying proteinuria as it has greater sensitivity
than a protein:creatinine ratio (PCR) for low concentrations of protein, and albumin is the predominant protein excreted
in the majority of proteinuric kidney diseases (see “Always quantify proteinuria when eGFR
or serum albumin are low”.25 Spot (random) urine samples are generally sufficient, although early morning
collection is preferable, as the sample will be more concentrated.25 Timed urine collection is not required
as spot sampling accurately reflects 24 hour albuminuria and proteinuria.25
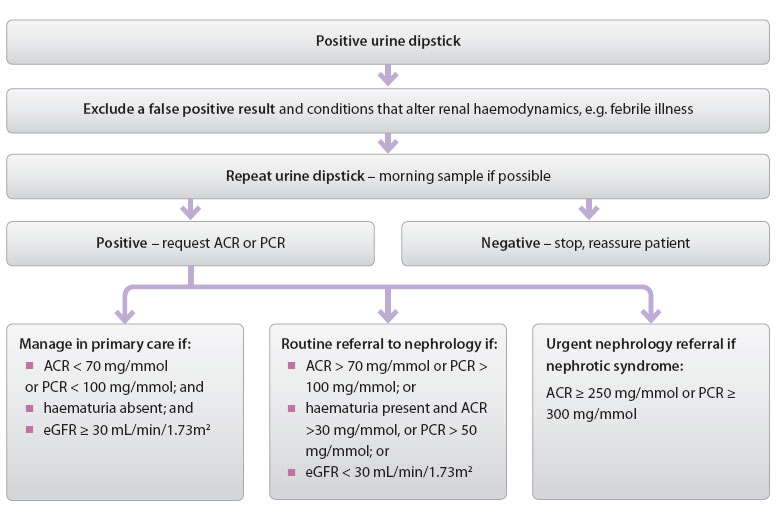
Figure 2: Investigating urine dipstick positive for proteinuria in primary care26
Major causes of persistent proteinuria
Table 2 provides guidance when considering causes for proteinuria in primary care.
Table 2: Causes of proteinuria that may be considered when assessing a positive dipstick7
| Common in primary care |
Transient/other |
Do not miss |
Consider |
- Diabetes
- Hypertension
- Obesity
- Medicines, e.g. NSAIDs
|
- Contamination by vaginal secretions
- UTI
- Orthostatic proteinuria
- Exercise
- Fever
|
- Congestive heart failure
- Glomerulonephritis
- Nephrotic syndrome
- Acute tubular damage
- Pre-eclampsia
|
- Congenital tubular disease, e.g. polycystic kidney disease
- Multiple myeloma
- Systemic lupus erythematosus (SLE)
- Myoglobinuria
- Haemoglobinuria
- Amyloidosis
|
Proteinuria and cardiovascular risk
People with CKD are at increased cardiovascular risk and are far more likely to die due to a cardiovascular cause than
they are of progressing to end-stage renal failure.11 A meta-analysis of 26 studies found evidence of a dose-response
relationship between albuminuria and the risk of coronary heart disease (CHD). 36 Individuals with microalbuminuria
had 50% greater risk of developing CHD and the risk in those with macroalbuminuria was increased more than 200%.36 This
study provides evidence that evaluation of proteinuria may be a useful future addition to cardiovascular risk assessment
in primary care. People with diabetes and over nephropathy (ACR ≥ 30 mg/mmol) are classified as having a five-year cardiovascular
risk greater than 20% and require intensive management to reduce risk factors.37
Follow-up investigations of confirmed proteinuria
Routine referral to nephrology is indicated for all patients with ACR > 70 mg/mmol or PCR > 100 mg/mmol.28
Urgent referral is required if nephrotic syndrome is suspected, i.e. proteinuria is in the nephrotic range (ACR ≥ 250
mg/mmol or PCR ≥ 300 mg/mmol), or if serum albumin is < 25 g/L, or oedema is present.29, 28 Patients with
haematuria and proteinuria (ACR > 30 mg/mmol or PCR > 50 mg/mmol) also require referral to nephrology.
If non-visible haematuria is present, a sample should be sent for urine microscopy.30 Red blood cell casts
and dysmorphic red blood cells are likely to be caused by glomerular disease.3,31 Non-glomerular causes of
proteinuria with haematuria include tubulointerstitial, renovascular or metabolic processes and generally occur without
red blood cell casts and dysmorphic red blood cells.31
Renal function should also be assessed and serum electrolytes measured.30 If eGFR is stable and ≥ 30 mL/min/1.732 ,
haematuria is absent and ACR < 70 mg/mmol, or PCR < 100 mg/mmol then the patient can be managed in primary care.28 If
eGFR < 30 mL/min/1.732 and haematuria is present the patient should be referred to nephrology regardless
of the level of proteinuria.28
Patients with proteinuria who are not referred to a Nephrologist should have blood pressure, urinalysis and renal function
assessed every 6 – 12 months.32 Hypertension should be treated to a target of less than 130/80 mmHg.33 Some
guidelines recommend a lower blood pressure target of 125/75 mmHg for the treatment of proteinuria, however, this target
should be approached with caution as a systolic target less than 120 mmHg is associated with an increased risk of adverse
events in people with diabetes.32, 33
IgA nephropathy and thin basement membrane disease
IgA nephropathy (Berger’s disease) is the most common form of primary glomerulonephritis. It is estimated to occur
in up to 6 – 10% of the general population, although many of these people may not present for medical care, so will remain
undiagnosed.19, 20 Peak incidence occurs in the second or third decade of life.21 In approximately
one-third of those affected, IgA nephropathy is characterised by episodes of visible haematuria coinciding with intercurrent
infections, usually of the upper respiratory tract (synpharyngitic haematuria), proteinuria, hypertension and progressive
renal dysfunction.22 Synpharyngitic haematuria is almost diagnostic of IgA nephropathy. A minority of people
with IgA nephropathy progress to end-stage kidney disease. As for all people with chronic kidney disease (CKD), the main
markers of progression are the presence and degree of proteinuria and development of hypertension. The degree of scarring
on renal biopsy strongly correlates with risk of progression. Treatment is aimed at blood pressure control, i.e. ACE
inhibitors and/or angiotensin-II receptor blockers (ARB), and reduction of proteinuria. Immunosuppression in IgA nephropathy
is controversial
Patients with IgA nephropathy who only have non-visible haematuria and no, or minimal, proteinuria, normal blood pressure
and normal renal function, have the same prognosis as the general population.
Thin basement membrane disease, also known as benign familial haematuria, is the most common reason for persistent
haematuria in children and adults.23 It is characterised by uniform thinning of the glomerular basement membrane
and mild proliferative glomerulonephritis.22 People with thin basement membrane disease often have lifelong
glomerular haematuria, but have minimal proteinuria and normal renal function. It is common for multiple family members
to be affected.22
Always quantify proteinuria when eGFR or serum albumin are low
Albumin comprises 60% of the body’s total plasma protein.38 It is the predominant protein excreted by people
with diabetes, hypertension and many glomerular diseases and is also a marker for disease progression.25 Urine
albumin quantification by ACR provides increased sensitivity and precision for detection of lower, but clinically significant
levels of protein than does total urine protein quantification via PCR.25 This is particularly important for
people with diabetes who are at increased risk of kidney disease.
It is recommended that all patients with an eGFR < 60 mL/min/1.73m2 have proteinuria quantified by measuring
ACR.25 In addition all patients require proteinuria quantification where there is a clinical suspicion of
nephrotic syndrome, e.g. serum albumin is low (hypoalbuminaemia).
The diagnostic criteria for nephrotic syndrome are:29
- ACR > 250 mg/mmol or PCR > 300 – 350 mg/mmol or proteinuria > 3 – 3.5 g/24 h
- Serum albumin < 25 g/L
- Clinical evidence of peripheral oedema
Request further testing if multiple myeloma is suspected
The index of suspicion for multiple myeloma should be increased in patients aged greater than 60 years with any bone
pain, and fatigue and/or weight loss, with or without hypercalcaemia.34 There may be accompanying laboratory
evidence of anaemia and renal impairment. Serum protein electrophoresis and serum-free light chain assay are recommended
by international guidelines when investigating suspected myeloma. Urine-free light chain assays are no longer considered
appropriate in this situation.35 A practical approach is to first request serum protein electrophoresis and
then if an increase in immunoglobulins is found, to discuss the need for further testing with a haematologist. Protein
dipstick is an inappropriate test to exclude multiple myeloma due to its inability to detect light-chain immunoglobulins.
Interpretation of leukocyte esterase and nitrites on dipstick in females
Urine dipstick testing is not required to diagnose a UTI, but in practice it is often performed and the presence or
absence of leukocyte esterase and nitrites can provide additional information.
Leukocyte esterase is an enzyme released by neutrophils and macrophages. A urine dipstick positive for this enzyme
indicates pyuria (an increased number of leukocytes). Urinary tract infections including cystitis and urethritis are
common causes of pyuria. Also consider sexually transmitted infections such as chlamydia. Pyuria is frequently associated
with haematuria, as both are symptoms of inflammation.39 The presence of leukocyte esterase on dipstick may
also be due to non-infectious renal diseases such as glomerulonephritis. Contamination of samples by vaginal secretions
may cause a false-positive result.
Nitrites are generally found in urine due to reduction of nitrates to nitrites by Gram-negative bacteria such as E.
coli. The detection of bacteria in urine by nitrite positive dipstick is also dependent on nitrates from the patient’s
diet (vegetables) and sufficient bladder incubation time. Gram positive uropathogens such as Staphylococcus saprophyticus and
Enterococcus do
not produce nitrate reductase and therefore when infection is due to these bacteria, the dipstick will be negative for
nitrite.
 Management of UTIs is not discussed in this article. For further information
see “Laboratory Investigation of UTI”, bpacnz, 2006.
Management of UTIs is not discussed in this article. For further information
see “Laboratory Investigation of UTI”, bpacnz, 2006.
How to collect and store urine samples
Clean-catch, midstream urine collection is the recommended method of collecting a sample for a urine dipstick test
in both males and females. It generally results in an uncontaminated sample, and there is no evidence that prior cleansing
of the external genitalia reduces contamination.3 If it is necessary to collect urine from patients with
an indwelling urinary catheter, a small quantity of initial urine should be drained and the collection drawn from the
sampling port.40
If further (laboratory) analysis of the sample is required, it should be appropriately labelled, and stored in a fridge
until collected. Analysis delays greater than two hours are reported to produce unreliable results.31
N.B. The nitrite dipstick reagent is sensitive to air exposure and containers of strips should be sealed whenever possible.31
 Best Practice Tip: Do not store blood and urine samples in the same bag. Even
small amounts of urine leakage can be drawn into the vacuum tube containing the blood specimen and contaminate it. Urine
specimens should be placed in a “ziplock” biohazard bag that is in turn placed in another biohazard bag with any other
samples from the patient. Printing separate forms for urine samples will encourage this practice.
Best Practice Tip: Do not store blood and urine samples in the same bag. Even
small amounts of urine leakage can be drawn into the vacuum tube containing the blood specimen and contaminate it. Urine
specimens should be placed in a “ziplock” biohazard bag that is in turn placed in another biohazard bag with any other
samples from the patient. Printing separate forms for urine samples will encourage this practice.