Infants who have not yet completed the National Immunisation Schedule and children who are not immunised, or only partially
immunised are most at risk from pertussis. The best way to protect at-risk individuals is on-time vaccination, as it
protects against infection for infants who are at highest risk and reduces the number of people in the community that
can transmit the bacteria. Pertussis booster vaccinations in the combination Tdap vaccine are fully-subsidised as of
January, 2013 for pregnant women between 28 – 38 weeks gestation
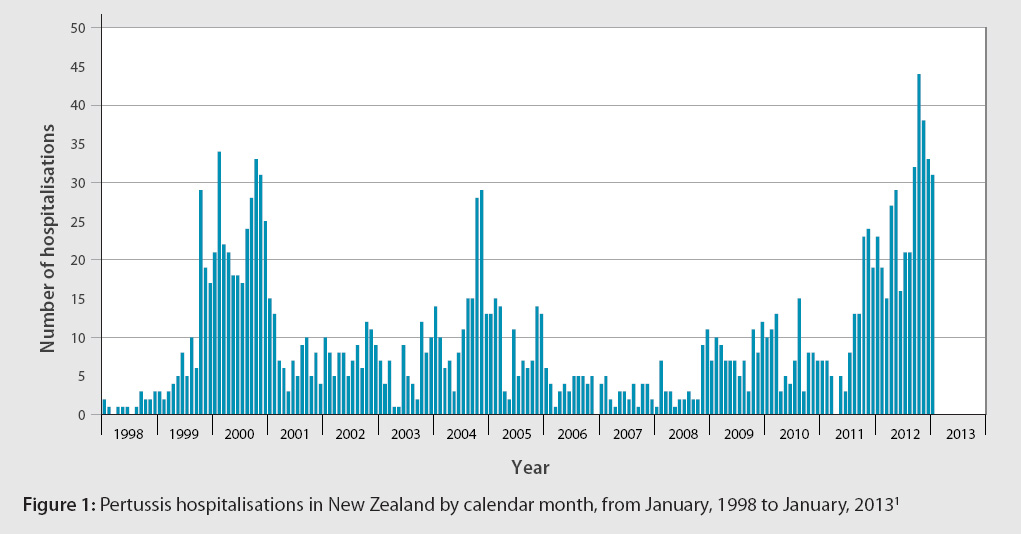
The New Zealand pertussis epidemic
Bordetella pertussis infection, also known as pertussis or whooping cough, is currently at epidemic levels in New
Zealand. In 2012 there were 5793 reported cases and two deaths.1 Canterbury (1209), Capital and Coast (680)
and Nelson Marlborough (670) DHBs reported the highest numbers.2 Figure 1 shows pertussis hospitalisations
by calendar month since 1998.
Infants aged under one year are at the greatest risk of severe disease and account for over 60% of the hospitalisations
that have occurred since the latest outbreak began in August 2011.2 Health professionals can reduce severe pertussis
infection rates by recommending on-time vaccination for all infants and children, and booster vaccinations for women
who are pregnant and adults with regular contact with infants.
Pertussis immunisation recommendations
There are three acellular pertussis-containing vaccines (i.e. vaccines containing only antigenic fragments of the
pertussis bacteria) funded under the New Zealand Immunisation Schedule:3
- INFANRIX-hexa provides protection against diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis and disease
caused by Haemophilis influenza type b. This vaccine is used for primary vaccinations in infants and children aged
up to seven years.
- INFANRIX-IPV provides protection against diphtheria, tetanus, pertussis and poliomyelitis. This vaccine is used
for primary vaccinations of infants and children aged up to seven years.
- BOOSTRIX or ADACEL is used to provide booster vaccinations against diphtheria, tetanus and pertussis. It is licensed
for use in children aged over ten years, adults and women who are pregnant.
In infants a primary course of INFANRIX-hexa is recommended at ages six weeks, three months and five months.3 The
minimum dose-interval is four weeks and the first dose is not recommended before age six weeks. A single dose of INFANRIX-IPV
is then recommended at age four years, followed by a booster at age 11 years with BOOSTRIX.3 The ideal coverage target
is for 95% of infants to be fully-vaccinated at ages six weeks and 16 months.3
Immunity to pertussis develops within 10 – 14 days of immunisation.3 However, the effectiveness of the pertussis vaccination
declines with time and protection can be expected to last between five to ten years in children.4 If pertussis vaccination
is administered after contracting a pertussis infection, the vaccination will be ineffective in preventing acute illness.
The Ministry of Health recommends that adults with regular contact with infants be offered booster vaccinations to
form a “cocoon of immunity” to protect infants in the first year of life. The effectiveness of this approach is likely
to be influenced by disease prevalence.5 During outbreaks, “cocooning” infants is more likely to reduce
the number of infants who become infected.5 The exact duration of protection from pertussis immunisation
in adults is unknown,3 but it can be presumed that vaccinations received in childhood are no longer providing
adequate protection from pertussis. All people with occupational (e.g. midwives, healthcare workers and carers) or household
(e.g. parents, grandparents and older siblings) contact with infants should receive a booster vaccination (BOOSTRIX
or ADACEL). The dose should be repeated every ten years for healthcare workers and people who work with children.3 Vaccination
is not subsidised in these groups.
Parents and grandparents may be more motivated to ensure they are immune to pertussis if it is explained that vaccination
will reduce the likelihood that they infect infants who have yet to complete the immunisation schedule. An international
study of 95 infants with pertussis found that 76 – 83% contracted pertussis from household members.6
Pertussis vaccination for pregnant women between 28 – 38 weeks gestation is now fully-subsidised (as of 1 January,
2013). This BOOSTRIX vaccination will be subsidised until the current pertussis epidemic is over and can be administered
at the same time as the influenza vaccination.
Maternal antibody levels have been shown to decrease quickly following pertussis immunisation, and levels in women
vaccinated during pre-conception or early in pregnancy may be insufficient to provide passive immunity to the newborn
infant.7 Therefore, immunisation later in pregnancy is more likely to provide some protection for the infant
while they are still vulnerable prior to completing their primary immunisation course.8 The vaccine can be safely given
to pregnant women from 20 weeks, and after 38 weeks gestation, but receiving the vaccine too early or too late may mean
that the infant is still exposed to pertussis during delivery and as a newborn. It takes approximately two weeks after
the booster vaccination for immunity to pertussis to develop. In addition, women 20 – 27 weeks or > 38 weeks gestation
would have to meet the costs of vaccination themselves.
The only contraindication for pertussis vaccination is an anaphylactic reaction to a previous dose,
or any component of the vaccine. Pertussis vaccination is not known to be associated with any significant adverse effects,
other than pain or redness at the injection site. Mild fever has been reported to occur in up to 20% of infants receiving
the vaccination.3 Vaccination of children with an evolving neurological disorder, e.g. uncontrolled epilepsy,
should be discussed with a paediatrician first.
Management of pertussis
The presentation of patients with pertussis is influenced by age and immunisation status. Young infants
may deteriorate rapidly and display apnoea and cyanosis, rather than cough. In older patients the illness often begins
with a seven to ten day period where symptoms are clinically indistinguishable from a minor respiratory infection. This
is referred to as the catarrhal stage, during which individuals are most infectious.3 Following the catarrhal
stage, paroxysmal bouts of coughing begin to develop, during which the characteristic “whoop” noise can often be heard
on inspiration in younger children. Gasping or gagging may be noticed instead of whooping in older children and adults.
Vomiting can follow bouts of coughing. Coughing lasts two to eight weeks and is often worst at night.
A diagnosis of pertussis during the catarrhal stage of the disease is difficult to confirm. A diagnosis
becomes more likely where a patient has an acute cough for 14 days or more and has either: an inspiratory whoop, paroxysmal
bouts of coughing, post-cough vomiting or apnoea, for which there is no other known cause.9
Laboratory testing is not required to confirm pertussis in an outbreak situation, where a patient is linked to a confirmed
case, for notification purposes or to confirm a patient is no longer infectious.9 Testing should be considered
where confirmation is needed to manage vulnerable contacts, e.g. where a family member is aged under one year, or where
a diagnosis is uncertain.9
Azithromycin subsidy and dosing changes

Azithromycin liquid (as of 1 November 2012) and tablets (as of 1 December 2012) are now fully subsidised, without
restriction, for a maximum of five days treatment. There is currently a lack of published consensus on the dosing regimen
for azithromycin in children for pertussis. United States, United Kingdom and Australian guidelines differ in their
advice, with some advising different regimens depending on age. The consensus in New Zealand, among infectious disease
specialists, is now to use the same regimen in children < 45 kg, regardless of age: 10 mg/kg on day one, followed by
5 mg/kg on days two to five. Due to concerns about resistance to azithromycin, it is not recommended as a first-line
treatment in adults for pertussis.
Treatment and prophylaxis of pertussis
Antibiotic treatment for pertussis is recommended to reduce transmission of the disease. Treatment should be initiated
if the patient presents within three weeks of onset of cough, after which time people are generally no longer infectious.9 Treatment
is unlikely to alter the clinical course of the illness, unless it is begun in the catarrhal stage.10 Antibiotics
should be given if the duration of cough is unknown. Women who are in the last trimester of pregnancy are considered
high-risk and should be prescribed antibiotics, regardless of the timing of onset.3 Young children can deteriorate
rapidly and may require hospitalisation.
Azithromycin is first-line for treatment and prophylaxis of pertussis in infants and children, erythromycin
is first-line for adults:
- Infants and children < 45 kg – azithromycin 10 mg/kg in a single dose on day one, followed by 5 mg/kg, once daily,
for days two to five (five days total). Erythromycin 10 mg/kg, four times daily for 14 days is an alternative.
- Children > 45 kg and adults – erythromycin 400 mg, four times daily for 14 days.* Azithromycin 500 mg on day one,
followed by 250 mg, once daily, on days two to five, is an alternative.
* Erythromycin ethyl succinate is currently the only subsidised form of oral erythromycin available in New Zealand.
Treatment and prophylaxis is recommended for 14 days with erythromycin ethyl succinate. There is evidence that seven
days of treatment with erythromycin estolate (which has superior tissue and serum concentrations compared with the other
erythromycin salts), is as effective as 14 days. However, erythromycin estolate is not currently available in New Zealand.11
Parents should be informed that the use of all macrolides (e.g. azithromycin or erythromycin) in infants aged under
three months is associated with an increased risk of hypertrophic pyloric stenosis and monitoring for complications,
e.g. forceful vomiting, should occur during treatment and for one month following completion.
Prophylactic antibiotics are recommended for people who have spent more than one hour in the close proximity of an
infectious person if they:9, 11
- Are aged less than one year
- Have an infant aged less than one year in the same household, or they spend significant time with infants aged less
than one year • Are pregnant, particularly in the last weeks of pregnancy
- Are at risk of severe complications, e.g. people who are immunocompromised or have severe asthma
 For further information see: “Pertussis:
an avoidable epidemic”, BPJ 45 (Aug, 2012).
For further information see: “Pertussis:
an avoidable epidemic”, BPJ 45 (Aug, 2012).
ACKNOWLEDGEMENT
Thank you to Dr Nikki Turner, Director, CONECTUS and The Immunisation Advisory Centre, University
of Auckland for expert guidance in developing this article.