In this article 
View / Download
pdf version of this article
Key concepts
- New Zealand has one of the highest rates of melanoma in the world
- Several factors can increase an individual’s risk of developing melanoma including; a large number of naevi
(moles), atypical naevi, frequent exposure to sun and sunburn during childhood and a family history of melanoma
- Early detection of melanoma involves clinicians being aware of the clinical signs of melanoma and algorithms that
can assist with diagnosis, e.g. ABCDE, Glasgow checklist
- Encourage patients to report any suspicious skin lesions and invite at-risk patients for periodic full-body skin
checks
- Refer, biopsy (depending on level of skill and clinical situation) or carefully follow up all suspicious skin lesions
- Dermatoscopy, digital photography and mole mapping are tools that can be used to aid in detection and surveillance
Melanoma in New Zealand
New Zealand has one of the highest rates of invasive melanoma in the world, with an incidence rate of approximately
41 per 100,000 people, per year.1,2 The most recently available data shows that in 2007, malignant melanoma
accounted for approximately 11% of all cancer registrations in New Zealand.3 Melanoma was the most commonly
registered cancer for males aged 25 to 44 years and the second most common for females in this age group and all people
aged 45 to 64 years.3
In New Zealand in 2007, 292 people died from malignant melanoma, making it the tenth most common cause of death from
cancer (accounting for 3.4% of all cancer deaths).3 The mortality rate from invasive melanoma is approximately
15%. Malignant melanoma is more common in males than females in New Zealand. Registrations are 16% higher and mortality
rate is 90% higher in males.3
Melanoma in Māori and Pacific peoples
Melanoma is significantly less common in Māori than in non-Māori people in New Zealand.3 There
is evidence that melanoma is also uncommon in Pacific peoples, with a combined incidence rate in 2004 for Māori and
Pacific peoples of 2.7 per 100,000 people, per year.2,4
Māori and Pacific peoples, however, present with melanomas that are thicker (>3 mm thickness) than those in non-Māori
people and therefore are likely to be at increased risk of death.4 It has been suggested that lesions at presentation
are thicker because Māori and Pacific peoples present late to primary care.2,4 However, evidence from
studies of melanoma in other darker skinned populations consistently show poorer outcomes and it is likely that a complex
mix of factors is responsible, including both biological (genetic and tumour type) and social aspects.5
In contrast to the non-Māori population in New Zealand, melanoma in Māori is more often found in women than
men.6 This female predominance is seen in other populations that have a low incidence of melanoma.7
In both Māori and non-Māori, melanoma is most frequently found on areas of the body that have been exposed
to the sun. In darker skinned populations in other parts of the world, approximately two-thirds of melanoma are found
on the palms, soles of the feet and under nails.5,6
Investigating lesions on the feet
The “CUBED” acronym can be used for investigating suspicious lesions on the feet.18 The presence
of any two features should trigger referral or excision. The “CUBED” acronym is:
- Coloured lesions where any part is not skin coloured
- Uncertain diagnosis
- Bleeding lesions on the foot or under the nail, including chronic granulation tissue
- Enlargement or deterioration of a lesion or ulcer despite treatment
- Delay in healing (> two months)
 Best Practice Tip: It can be difficult to determine the cause of subungual
bleeding. Always ask about a history of trauma to the nail. An area of clear nail growth will develop at the base of
the nail with time (weeks) if the subungual bleeding is a result of trauma (and not if the lesion is melanoma).18
Best Practice Tip: It can be difficult to determine the cause of subungual
bleeding. Always ask about a history of trauma to the nail. An area of clear nail growth will develop at the base of
the nail with time (weeks) if the subungual bleeding is a result of trauma (and not if the lesion is melanoma).18
What is malignant melanoma?1
Melanoma is a type of skin cancer that develops from melanocytes (melanin-producing cells), which are normally located
in the basal layer of the epidermis or within the dermis. Although melanoma is less common than non-melanoma skin cancers,
e.g. basal cell carcinoma and squamous cell carcinoma, it causes far more deaths. Melanoma may develop within an existing
melanocytic naevus but more often arises from skin that appears normal. Melanoma can occur anywhere on the skin and on
rare occasions, in other tissues such as the eye, central nervous system and the mucosa of the gastrointestinal, genitourinary
and respiratory systems. Aggressive forms of melanoma can metastasise to almost any organ in the body.
What is a mole?1
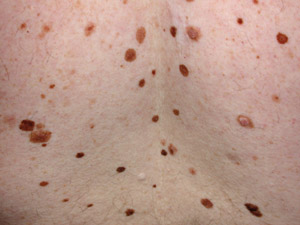
A mole (melanocytic naevus) results from benign proliferation of melanocytes. Most European New Zealanders have 20–50
moles, depending on genetics and exposure to the sun. Some moles are congenital and others are acquired, particularly
during childhood and adolescence. Development of a new mole later in life, e.g. after age 50 years, is less common.
An atypical naevus (also called Clark’s naevus) is a melanocytic naevus that is unusual in appearance – i.e.
a “funny-looking mole” (Figure 1). Most atypical naevi develop during childhood. Generally people, particularly
those with fair skin, have up to ten atypical naevi however some with a familial syndrome may have several hundred. A
person with five or more atypical naevi is at higher risk of melanoma. However, an individual atypical naevus has very
low risk of malignant change as most melanoma arise de novo.
A mole with three or more of the following clinical features can be defined as atypical:
- Size that is > 5 – 6 mm in diameter
- Border that is blurry or ill-defined
- Unusual irregular shape
- Variation in colour
- Variation in profile i.e. flat and raised parts
 For further information see: www.dermnet.org.nz
For further information see: www.dermnet.org.nz
Risk factors for melanoma
Screening for melanoma at a population level is not indicated but raising awareness of the factors that increase individual
risk is important for both patients and clinicians.
The following factors increase the risk of melanoma.
Age: The risk of melanoma increases with age, particularly over age 60 years.2,8 Incidence
is higher in older men who tend to present with melanoma that are thicker and therefore are more at risk of metastasis.9 Although
melanoma is rare in children (estimated to be 1–2% of all melanoma cases), there is evidence that they may present
with thicker lesions.10 This has been attributed to a higher incidence of atypical lesions and a possible delay
in presentation and diagnosis, due to the rarity of melanoma in this age group.11
Past history of melanoma or non-melanoma skin cancer: A person with a history of melanoma has a greater
than ten times increased risk of developing another melanoma.7,12 A past history of non-melanoma skin cancer
increases the risk around four-fold.9
Family history of melanoma: A history of melanoma in a first degree family member almost doubles an
individual’s risk of developing melanoma.9 It is estimated that approximately 10% of people with melanoma
have a family history of melanoma in a first degree relative.13 This increased risk may be attributed to, in
part, by behavioural and environmental factors that are shared by these families but it is likely that there are also
underlying genetic factors.13
Number of moles: The risk of melanoma increases as the number of melanocytic naevi (moles) increases,
particularly if there are more than 100.1,7
Type of moles:The risk of melanoma increases if the patient has atypical naevi, with a six-fold increase
in risk for those with more than five.1,7,9
Ultraviolet radiation: Exposure to ultraviolet radiation from the sun (UVB, UVA) or from sun bed use
(UVA) increases the risk of melanoma.8 Frequent exposure to sun and sunburn during childhood and adolescence
approximately doubles the risk of melanoma. Ongoing exposure in adulthood also contributes to risk, particularly if the
exposure is intermittent in a person unaccustomed to the sun.7,8 However, a history of sunburn has limited
clinical predictive value in regions such as New Zealand where the prevalence of sunburn is high.9
Skin type: Skin type can be categorised by response to UV exposure. Table 1 describes the six Fitzpatrick
phototypes and the response of each type of skin to UV exposure.1 The paler the skin, the more likely it is
to burn and therefore the more protection it needs from UV radiation.
| Table 1: The Fitzpatrick skin types (adapted from Dermnet
NZ)1 |
| Skin type |
Typical Features |
Response to UV exposure |
I
|
Pale white skin, blue/hazel eyes, blonde/red hair |
Always burns, does not tan |
| II |
Fair skin, blue eyes |
Burns easily, tans poorly
|
III
|
Darker white skin |
Tans after initial burn |
| IV |
Light brown skin |
Burns minimally, tans easily |
| V |
Brown skin |
Rarely burns, tans darkly easily
|
| VI |
Dark brown or black skin |
Never burns, always tans darkly
|
Gender: Males (non-Māori) in New Zealand and in other countries with a high-incidence of melanoma,
e.g. Australia, United States, are at higher risk of melanoma than females. This is in contrast to other areas in the
world with lower incidence of melanoma, e.g. Germany, Mediterranean counties, where there is usually a female predominance.2,14 Although
the number of Māori with melanoma is low, there is evidence that Māori females are twice as likely to have melanoma
as Māori males.6
Men are more likely to have melanomas on the trunk (approximately 40%) and women more likely to have lesions on the
legs (approximately 35%).1,15
Geographical location: New Zealand’s geographical location increases the risk of melanoma for
several reasons, including :2,16
- Large number of fair-skinned people
- Mild climate allowing outdoor activities during the middle of the day
- Changeable weather resulting in intermittent rather than constant sun exposure, which may alter people’s sun-seeking
behaviour and potentially increase the risk of sunburn
- 40% higher levels of peak summer UV radiation compared to countries with similar latitude and altitude in Northern
hemisphere
Incidence rates of melanoma also vary throughout New Zealand with lower rates reported in the Southland area (approximately
20 cases per 100,000 people per year) compared to the Taranaki area (approximately 70 cases per 100,000 people per year).2 The
reasons for this difference are not clear but are likely to relate to atmospheric factors.
Types of melanoma
There are five main types of melanoma, distinguished by their clinical appearance, the growth and behaviour of the
lesion and their location on the body. Approximately 10% of melanomas are non-pigmented (amelanotic) and this increases
the difficulty in making an accurate diagnosis.7 Once invasive, all types of melanoma can metastasise.
In addition to the five main types of melanoma there are a number of other more unusual types, such as desmoplastic
melanoma and malignant blue naevus. Combinations of melanoma can also occur, such as a nodular melanoma growing within
a superficial spreading melanoma.1 Cutaneous metastatic melanoma may present as blue-black to pink-red solitary
or multiple firm nodules. They may be close to the site of primary melanoma (satellites) or distant due to haematogenous
spread. In some patients with metastatic melanoma, the site of the primary lesion may be unknown. Enquire about a history
of skin lesion excision.
The five main clinical types of primary cutaneous melanoma are pictured.1,7,17 (Images provided by DermnetNZ) |
| 1. |
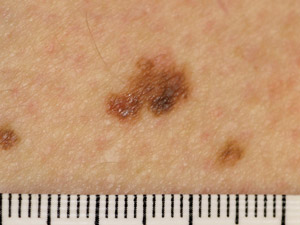 Superficial
spreading melanoma (SSM) – the most common type of melanoma. Usually found on areas of the body which
have been exposed to the sun. This type of melanoma is typically a flat patch that is irregularly shaped, irregularly
pigmented and has an irregular outline. SSM frequently has a prolonged pre-invasive in-situ phase, growing slowly over
months to years Superficial
spreading melanoma (SSM) – the most common type of melanoma. Usually found on areas of the body which
have been exposed to the sun. This type of melanoma is typically a flat patch that is irregularly shaped, irregularly
pigmented and has an irregular outline. SSM frequently has a prolonged pre-invasive in-situ phase, growing slowly over
months to years |
| 2. |
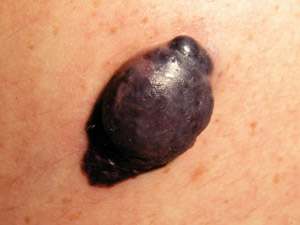 Nodular
melanoma (NM) – this is the second most common type of melanoma and presents as a rapidly-growing (over
several weeks to months) pink, red, brown or black nodule. The pigmentation within NM is often more uniform than in
SSM. It may arise within an existing melanocytic naevus or in normal appearing skin. NM may be more likely to bleed
or ulcerate than SSM. NM does not have an in-situ phase. Nodular
melanoma (NM) – this is the second most common type of melanoma and presents as a rapidly-growing (over
several weeks to months) pink, red, brown or black nodule. The pigmentation within NM is often more uniform than in
SSM. It may arise within an existing melanocytic naevus or in normal appearing skin. NM may be more likely to bleed
or ulcerate than SSM. NM does not have an in-situ phase. |
| 3. |
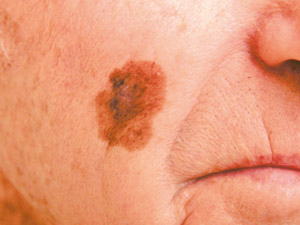 Lentigo
maligna melanoma (LMM) – this type of melanoma is found on sun-damaged skin in older people, e.g. on
the head and neck. The lesion typically has a long pre-invasive in-situ stage (years to decades), termed lentigo maligna
(LM). LM presents as an enlarging, irregularly pigmented freckle. Lentigo
maligna melanoma (LMM) – this type of melanoma is found on sun-damaged skin in older people, e.g. on
the head and neck. The lesion typically has a long pre-invasive in-situ stage (years to decades), termed lentigo maligna
(LM). LM presents as an enlarging, irregularly pigmented freckle. |
| 4. |
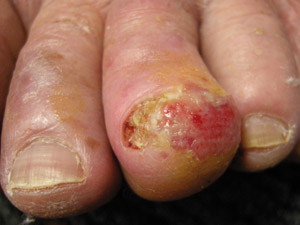 Acral
lentiginous melanoma (ALM) – this type of melanoma is found on the palms of the hands, soles of the
feet and underneath the finger or toe nails. ALM account for less than 5% of all melanomas in New Zealand. Although
still rare, ALM is the most common type of melanoma in dark-skinned people (Fitzpatrick phototype 5 or 6). Melanoma
on the feet may not always demonstrate the characteristics associated with melanoma at other body sites and there is
a higher rate of amelanotic (non-pigmented) melanoma among acral lesions (see sidebar: “Investigating
lesions on the feet”).18 Acral
lentiginous melanoma (ALM) – this type of melanoma is found on the palms of the hands, soles of the
feet and underneath the finger or toe nails. ALM account for less than 5% of all melanomas in New Zealand. Although
still rare, ALM is the most common type of melanoma in dark-skinned people (Fitzpatrick phototype 5 or 6). Melanoma
on the feet may not always demonstrate the characteristics associated with melanoma at other body sites and there is
a higher rate of amelanotic (non-pigmented) melanoma among acral lesions (see sidebar: “Investigating
lesions on the feet”).18 |
| 5. |
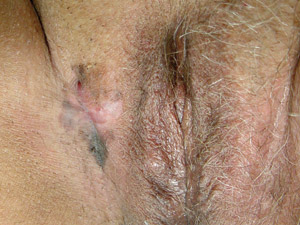 Mucosal
lentiginous melanoma (MLM) – these lesions arise from mucosal or paramuscosal sites including the vulva,
vagina, penis, anus, eyelids, conjunctiva, oral cavity or lips. Mucosal
lentiginous melanoma (MLM) – these lesions arise from mucosal or paramuscosal sites including the vulva,
vagina, penis, anus, eyelids, conjunctiva, oral cavity or lips. |
Early detection of melanoma improves survival
Early detection of melanoma results in better outcomes for patients. The challenge for GPs is to accurately recognise
skin lesions that are suspicious. Early detection also relies on the patient being aware of the need to have skin lesions
checked. If a suspicious skin lesion is identified further treatment is essential. Depending on the level of clinical
suspicion this may be immediate referral, biopsy or accurate documentation of the lesion and organised follow-up.
How can GPs help ensure melanoma is detected early?
- Be aware of the clinical signs of melanoma and algorithms that can assist with diagnosis, e.g. ABCDE, Glasgow checklist
(See below)
- Be familiar with which patients are at increased risk of melanoma
- Educate patients who are at increased risk of melanoma about the clinical signs to watch for and encourage them to
examine their skin monthly (see sidebar: “Skin self-examination”)
- Consider periodically asking all patients if they are concerned about any moles or skin lesions, and offer full skin
checks to older males
- When examining a patient for another clinical problem take note of any skin lesions with an unusual appearance (an “ugly
duckling”, See below) or scars from previous excisions of suspicious lesions
- A check of a single lesion that is of concern can be done quickly during a consultation for another clinical problem,
although the entire skin surface should be examined if indicated, e.g. in high risk patients. Ensure access to good lighting
when examining the skin.
- Refer, biopsy (depending on level of skill and clinical situation) or carefully follow up all suspicious skin lesions
- If a biopsy is taken, ensure that the results are followed up, e.g. place a recall or reminder in the patient notes
- Monitor clinically doubtful skin lesions for one to two months (but no more than three months).9 Consider
the use of digital photography to monitor changes. Ensure the patient knows to re-present sooner if there is any concern.
- Clinical photography with macroscopic and dermoscopic views can enable a second opinion from an expert (teledermoscopy)
- Consider taking a training course in dermatoscopy
Skin self-examination
Self-examination of the skin should be seen as complementary to a skin examination by a health professional.12 The
aim of self-examination is for the patient to detect suspicious lesions early so that they present for a skin check by
a doctor to enable reassurance, observation, excision or referral as appropriate.
A full self-examination of the skin requires assistance from another person or the use of two mirrors. A hair dryer
can be useful when examining the scalp.
 A guide to skin self examination can be found at: www.cancernz.org.nz Search
term: Skin check
A guide to skin self examination can be found at: www.cancernz.org.nz Search
term: Skin check
Provide information about melanoma
Although mortality from melanoma decreases with early detection, some patients will still die from melanoma. Public
health education and media reports have increased awareness about melanoma, however, some patients still do not seek medical
advice at an early enough stage.12 Where practical, information can be provided to help patients determine
normal from abnormal moles.19 Validate the usefulness of a skin lesion check as patients may feel that this
is a trivial reason for visiting a GP.19
Encourage patients to report rapidly growing lesions
Some types of melanoma, e.g. nodular melanoma, grow very rapidly and are biologically aggressive from the outset.12 Some,
e.g. amelanotic melanomas, are more difficult to recognise because they do not usually display the classical clinical
features.20 Make patients aware that any rapidly growing or odd looking lesions should be checked. A few months
of rapid growth may adversely affect prognosis.
Offer high risk patients a full-body skin check
Practical issues such as a lack of time, competing co-morbidities and patient embarrassment may limit the extent of
a skin examination,21 increasing the chance that lesions will not be detected. Population based screening using
full-body skin examination is not recommended as there is no clear evidence that this is effective in reducing mortality.9,12 However,
patients who are at high risk of melanoma should be offered a full-body skin examination, that includes the scalp and
skin folds. New Zealand guidelines recommend six-monthly full body skin examinations in high risk patients.9 Patients
may need to request a longer consultation time for a full body skin check or return for an additional consultation, particularly
if they have other issues to discuss.
Although self-examination of the skin is beneficial and should be encouraged, there is evidence that full body skin
examinations by doctors detect more melanomas than self-examination and also that the lesions found are more likely to
be thinner or melanoma in situ.22,23 In a study of well-motivated patients only 55% were continuing to examine
their skin after one year.22
The use of clinical checklists may facilitate early detection of melanoma
Checklists of clinical features have been developed to assist in the detection of suspicious skin lesions by both patients
and clinicians. Becoming familiar with the three most frequently used tools outlined below is advantageous, because a
combined approach is most useful to detect malignant lesions.
The ABCDE criteria
Any combination of the ABCDE criteria may indicate a suspicious lesion:24,25
- Asymmetry – one half of the lesion does not match the other
- Border irregularity – notched, blurred or ragged edges
- Colour variegation - different colours such as brown, black, white, red or blue within the same lesion
- Diameter greater than 6 mm – the majority of melanoma are more than 6 mm in size although up
to 25% of new lesions may be smaller
- Evolution or Enlargement – any change in a lesion over time is suspicious (colour, shape or
symptoms)
The disadvantage of the ABCDE criteria is that they are not very specific. Other types of skin lesions such as seborrheic
keratoses can also exhibit the same features and early melanoma may not initially display these clinical characteristics.20 In
addition, not all skin lesions that change are melanoma. Moles undergo symmetrical enlargement, particularly in younger
patients as they grow. Some skin lesions darken in a uniform manner after sun exposure and trauma or chronic rubbing may
cause changes in colour or texture.20
The Glasgow seven point checklist
This checklist includes major and minor clinical features which are assigned a score. The checklist is:7,26
Major features (two points)
- Change in size
- Change in shape (irregular border)
- Change in colour (irregular pigmentation)
Minor features (one point)
- Inflammation
- Crusting, oozing or bleeding
- Sensory change or itch
- Lesion diameter ≥ 6 mm
Specialist referral or excision is indicated for patients with skin lesions which score three points or more.27 Referral
or excision should also be considered in the presence of any one clinical feature when there is a strong suspicion of
melanoma, as major features are not always present.7,27 The checklist is more complex and therefore less widely
adopted than the ABCDE criteria.
The “ugly duckling” sign
The underlying clinical rationale for the “ugly duckling” sign is that most naevi in an individual are similar
in appearance, therefore a lesion that is not like others should receive special attention, even though it may not raise
suspicion on the basis of other clinical tools. The tool aims to improve specificity (i.e. reduce the number of benign
lesions that are removed) but not decrease sensitivity (i.e. not miss detecting melanoma). It is most useful in patients
with large numbers of atypical naevi and may reduce the number of unnecessary resections. For example, in a patient with
multiple but similar atypical lesions, e.g. red-brown with irregular borders, the “ugly duckling” lesion for
that patient may be a small, well-defined black lesion.28
Dermatoscopy and digital technology
Dermatoscopy and forms of digital technology such as digital dermatoscopy or photography are additional tools that may
be used by experienced clinicians to aid early diagnosis of melanoma.
Dermatoscopy (also called dermoscopy) is the technique of examination of the skin using a magnifying
lens (usually 10×) and a light source. Modern dermatoscopes are small, portable, hand held devices that are used extensively
in specialist practice and increasingly in primary care. When used by a trained clinician, dermatoscopic examination can
reduce unnecessary excisions of benign skin lesions and increase the accuracy with which early melanoma is detected.29
Two forms of dermatoscopic systems are available:1
- Fluid immersion systems where the lens is placed in contact with the skin and oil or alcohol is used to eliminate
any light reflections from the skin. Contact dermatoscopy gives a better quality image but has the disadvantage of compression
of vascular structures.
- Polarised light non-contact systems which give better images of deeper structures and the vasculature. They do not
require an immersion fluid and multiple lesions can be quickly viewed.
Effective dermatoscopy requires initial training, ongoing learning experience and access to specialist advice. Digital
macro and dermatoscopic photography of suspicious lesions is highly recommended because it:
- Enables binocular evaluation of an enlarged view
- Enhances recognition of global and local dermatoscopic features
- Increases self-learning
- Allows more accurate follow-up than by memory and measurement alone
- Is easy to obtain a second opinion
Digital photography may be used to record the position and characteristics of skin lesions on a patient’s
whole body. The images can then be repeated over time to detect new lesions and lesions that may have changed in shape,
size or colour.
Dermatoscopic images can be obtained and repeated over one to three months to assist in the follow
up of suspicious skin lesions. For longer term surveillance, images can be repeated six to 12 monthly.
Mole mapping refers to a range of techniques that record the position and characteristics of skin lesions
on the body. In its simplest form it may be the recording of lesions on a hand drawn figure. The use of digital photography,
dermatoscopic images and computer software allowing serial monitoring has developed mole mapping to a new level. Mole
mapping when used for surveillance combines digital technologies with risk assessment, patient education and regular specialist
follow-up.1 Mole mapping is most useful in patients with a large number of moles (> 50–100), atypical
naevi, moles on the back that are hard to see and also in patients who are at increased risk of melanoma.1
The advantages and disadvantages of mole mapping (Table 2) should be discussed with the patient. A mole map is a diagnostic
service and if a suspicious lesion is identified the patient is referred back to their GP or specialist for further treatment.
In New Zealand, private mole mapping services, including assessment of the images by a dermatologist are available throughout
the country, e.g. www.molemap.co.nz.
| Table 2: Advantages and disadvantages of mole mapping (adapted from Dermnet
NZ)1 |
| Advantages |
Disadvantages |
Rapid assessment of change in a lesion
|
May miss melanoma in areas such as the scalp and genitals |
Lesions of concern are detected early allowing careful follow up or early referral
for surgery
|
False negatives (early melanoma may look benign and be missed) |
Unnecessary excisions may be reduced
|
False positives (benign lesions may be excised unnecessarily) |
Access to mole mapping may be quicker and easier than access to a dermatologist
|
The interval between mole mapping appointments may be too long for rapidly growing
lesions |
| Patient and doctor reassurance |
Cost (approximately $300) |
The role of biopsy
Indications for excision of a suspicious skin lesion include:
- Lesion with typical clinical / dermoscopic features of melanoma
- Solitary atypical naevus (e.g. > 6 mm, irregular shape, asymmetry of structure and colour) in a site that is difficult
to observe
- Atypical flat naevus that has been objectively observed to enlarge, e.g. by photographic comparison or observation
by a reliable witness
- Enlarging pigmented or red nodule, particularly if symptomatic or if it is not possible to confidently diagnose a
benign lesion such as dermatofibroma
Flat lesions can safely be observed if clinical concern is minimal.
If biopsy is indicated, an excision biopsy of the complete lesion with a 2 mm rim of normal skin and a cuff of fat is
recomended.7,9 The depth of the excision biopsy should extend to the deep fascia. This method provides sufficient
material for histological examination and does not compromise a wider excision if required.7
Punch biopsy or other forms of partial biopsy of suspicious skin lesions is not recommended because it may not give
sufficient material for an accurate pathological assessment of the lesion.7,9 A partial biopsy or multiple
biopsies may be appropriate in clinical situations such as a large facial lesion or acral lesion. However, expert advice
should be obtained first.9 Biopsy can also be useful to differentiate the lesion from a non-melanocytic tumour
such as seborrhoeic keratosis. If it reveals a melanocytic lesion, particularly if there is atypia, complete excision
should then be undertaken. The risk of seeding or dissemination of the melanoma with a partial biopsy is no longer thought
to be significant.9
 Best Practice Tip: Histological diagnosis of melanoma is often
difficult. Provide the pathologist with a careful description of the lesion and explain why it is suspicious. Some pathologists
find clinical photographs useful. Draw attention to areas of specific concern, e.g. eccentric pigmentation, as melanoma
might be arising within an otherwise benign lesion. One way of doing this is by using a biopsy punch to score the skin
around the suspicious area.
Best Practice Tip: Histological diagnosis of melanoma is often
difficult. Provide the pathologist with a careful description of the lesion and explain why it is suspicious. Some pathologists
find clinical photographs useful. Draw attention to areas of specific concern, e.g. eccentric pigmentation, as melanoma
might be arising within an otherwise benign lesion. One way of doing this is by using a biopsy punch to score the skin
around the suspicious area.
Sun protection and melanoma
Research to demonstrate the protective effect from sunscreen against melanoma has been difficult and at times controversial
for several reasons including:30
- Individuals at higher risk of sunburn are at higher risk of melanoma
- Individuals at higher risk of sunburn are more likely to use sunscreen
- Sunscreen use may result in longer periods of sun exposure
- Previously the majority of sunscreens protected against UVB but not UVA (most sunscreens now provide protection from
both)
The risk of melanoma can be reduced with avoidance of the sun at times of high UV levels and sun protective measures
such as using sunscreen and wearing a hat and clothing that covers the skin. The “slip, slop, slap and wrap message” should
continue to be promoted (see sidebar: “How to be SunSmart”).31
Sun exposure, vitamin D and melanoma
Minimal exposure to the sun may result in a deficiency of vitamin D levels as UVB radiation is required for the production
of vitamin D3 within the skin. Ideally, a balance can be achieved between safe levels of sun exposure and sufficient sun
exposure to maintain adequate vitamin D levels. New Zealand guidelines recommend that short episodes of sun exposure,
i.e. six to eight minutes in the summer and six to 50 minutes in the winter, depending on skin type and latitude, to 15–20%
of the body most days, is required to maintain vitamin D levels.9 Supplementation with vitamin D is likely
to be safer than sun exposure for people who are at higher risk of melanoma, e.g. elderly people, people in residential
care, people with a past history of melanoma or who are very sensitive to the sun and people who are on medications that
increase photosensitivity.
How to be SunSmart
Encourage patients to be “SunSmart”. In the middle of the day in summer it can take only 15 minutes for
fair skin to burn. A wide range of information sheets on sun protection and skin cancer are available on the New Zealand
Cancer society website: www.cancernz.org.nz
- Avoid exposure to the sun between 11 am and 4 pm, especially from September to March when UV levels
are high. Also avoid sun beds.
- Slip on sun-protective clothing
- Slop on sunscreen that is broad-spectrum and SPF 30+ and reapply after every two hours in the sun
and after swimming
- Slap on a hat
- Seek shade
- Wrap on some good quality sunglasses
Acknowledgement
Thank you to Dr Amanda Oakley, Specialist Dermatologist and Clinical Associate Professor, Tristram
Clinic, Hamilton for expert guidance in developing this article.
References
- Dermnet NZ. Clinical Topics A-Z. Dermatological Society of New Zealand, 2010. Available from: www.dermnet.org.nz (Accessed
Jan, 2011).
- Liang J, Robinson E, Martin R. Cutaneous melanoma in New Zealand: 2000-2004. A NZ J Surg 2010;80:312-16.
- Ministry of Health. Mortality and demographic data 2007. Wellington: Ministry of Health, 2010.
- Richardson A, Fletcher L, Sneyd M, et al. The incidence and thickness of cutaneous malignant melanoma in New Zealand
1994-2004. N Z Med J 2008;121(1279):18-26.
- Kabigting D, Nelson F, Kauffman C, et al. Malignant melanoma in African-Americans. Dermatology Online Journal 2009;15(2):3.
- Hore T, Robinson E, Martin R. Malignant melanoma amongst Māori and New Zealand Europeans, 2000-2004. World J
Surg 2010;34:1788-92.
- Scottish Intercollegiate Guidelines Network (SIGN). Cutaneous Melanoma. SIGN, 2003. Available from: www.sign.ac.uk (Accessed
Jan, 2011).
- Curiel-Lewandrowski C. Risk factors for the development of melanoma. UpToDate 2010. Available from: www.uptodate.com (Accessed
Jan, 2011).
- Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical practice guidelines for the management
of melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New
Zealand Guidelines Group. Wellington, 2008. Available from: www.nzgg.org.nz (Accessed
Jan, 2011).
- Berk D, LaBuz E, Dadras S, et al. Melanoma and melanocytic tumours of uncertain malignant potential in children,
adolescents and young adults – The Stanford Experience 1995-2008. Pediatr Dermatol 2010;27(3):244-54.
- Lange J, Palis B, Chang D, et al. Melanoma in children and teenagers: an analysis of patients from the national cancer
data base. J Clin Oncol 2007;25(11):1363-8.
- Psaty E, Scope A, Halpern A, Marghoob A. Defining the patient at high risk for melanoma. Int J Derm 2010;49:362-76.
- Kasparian N, McLoone J, Meiser B, et al. Skin cancer screening behaviours among individuals with a strong family
history of malignant melanoma. Br J Cancer 2010;103:1502-9.
- Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol 2009;27:3-9.
- Salmon P, Chan W, Griffin J, et al. Extremely high levels of melanoma in Tauranga, New Zealand: Possible causes and
comparisons with Australia and the northern hemisphere. Australas J Derm 2007;48:208-16.
- National Institute of Water and Atmospheric Research (NIWA). UV & Ozone. Available from: www.niwa.co.nz/our-science/atmosphere/our-services/uv-and-ozone (Accessed
Jan, 2011).
- Diagnostic Medlab. Melanoma and recommendations for excision margins of cutaneous malignancies. Available from: www.dml.co.nz (Accessed
Dec, 2010).
- Bristow I, de Berker D, Acland K, et al. Clinical guidelines for the recognition of melanoma of the foot and nail
unit. J Foot Ankle Res 2010;3:25.
- Walter F, Humphrys E, Tso S, et al. Patient understanding of moles and skin cancer, and factors influencing presentation
in primary care: a qualitative study. BMC Fam Pract 2010;11:62.
- Goodson AG, Grossman D. Strategies for early melanoma detection: Approaches to the patient with nevi. J Am Acad Dermatol
2009;60:719-35.
- Olivera S, Heneghan M, Cushman L, et al. Skin cancer screening by dermatologists, family practitioners, and internists.
Barriers and facilitating factors. Arch Dermatol 2011;147(1):39-44.
- Kantor J, Kantor D. Routine dermatologist-performed full-body skin examination and early melanoma detection. Arch
Dermatol 2009;145(8):873-6.
- Epstein D, Lange J, Gruber S, et al. Is physician detection associated with thinner melanomas? JAMA 1999;281(7):640-3.
- Friedman R, Rigel D, Kopf A. Early detection of malignant melanoma: the role of physician examination and self-examination
of the skin. CA Cancer J Clin 1085:35:130-51.
- Rigel D, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA: A Cancer J Clin
2010;60:301-16.
- MacKie R. Clinical recognition of early invasive malignant melanoma. BMJ 1990;301:1005-6.
- Clinical Knowledge Summaries. Skin cancer – suspected. Available from: www.cks.nhs.uk/skin_cancer_suspected (Accessed
Jan, 2011)
- Grob J, Bonerandi J. The ‘Ugly Duckling’ sign: Identification of the common characteristics of nevi in
an individual as a basis for melanoma screening. Arch Dermatol 1998;134:103-4.
- Lee J, Hirokawa D. Dermatoscopy: facts and controversies. Clin Dermatol 2010;28:303-10.
- Green A, Williams G, Logan V, Strutton G. Reduced melanoma after regular sunscreen use: Randomized trial follow-up.
J Clin Oncol 2010;[Epub ahead of print].