In this article 
View / Download
pdf version of this article
Key reviewer: Dr Peter Fitzgerald, Cytopathologist, Southern Community Laboratories Ltd, Dunedin
| Keypoints |
- The two Liquid Based Cytology (LBC) systems currently used in New Zealand are SurePath and ThinPrep. Both systems
use different collection vials and collection devices, which are not interchangeable between the two systems
- Wooden spatulas should not be used for LBC. If using a spatula it must be plastic
- HPV testing is not indicated in women less than 30 years of age due to the high prevalence of HPV infection in
young women, the vast majority of which clear within 2 years and are of little clinical significance
- A positive HPV test in women older than 30 years of age indicates increased risk of developing a high grade lesion,
and so can be a useful adjunct to the management of abnormal cellular changes
|
Liquid-based cytology (LBC) and Human Papillomavirus (HPV) testing have recently been adopted to aid with the collection,
diagnosis and management of cervical cytology.
The main advantages of LBC are a reduction in the rates of unsatisfactory smears, shorter time required for interpretation,
and the ability to use the same sample for HPV testing.
Introduction of liquid based cytology for cervical smears
Cervical screening started in New Zealand the 1950s, then in 1991 the National Cervical Screening Programme (NCSP) was
launched. Since then the vast majority of cervical smears collected over the last 50 years have been performed by the
traditional Pap smear method of cells being spread onto a glass slide and a fixative used to prevent air drying.
Liquid based cytology has been available in New Zealand for approximately 10 years. This has had variable uptake most
likely due to the additional surcharge that was previously passed on to the patient. Now that LBC has become the method
of choice for cervical cell cytology, it is being rolled out throughout New Zealand at no cost to practice or patient.
LBC represents the first major change in preparation method for cervical screening samples for over 50 years. Instead
of cells being smeared onto a glass slide, they are washed into a vial of fixative. Then at the laboratory a random sample
of cells is presented in a thin layer on a glass slide. These slides can then either be screened in the usual manner or
subjected to partially automated imaging. The process is being widely used in the United States, the UK and in many European
countries, where they no longer routinely collect conventional Pap smears.
The National Cervical Screening Programme has agreements in place for gynaecological cytology with a number of providers
around New Zealand. All providers will be using liquid based cytology for cervical smears by the end of 2009.
Why the change?
There are number of reasons that have led to the adoption of liquid based cytology in New Zealand.
1. The strong influence of international research and laboratory trends
Most research has concluded LBC is at least as sensitive as conventional Pap smear with the advantage of a reduction
of unsatisfactory slides. LBC for cervical smears has become the method of choice in a number of countries.
2. The introduction of the “Guidelines for Cervical Screening in New Zealand” (2008)1
The guidelines state cervical smears may be collected by either conventional Pap smear or by LBC, but they acknowledge
there may be situations where liquid-based cytology offers some advantage over conventional smears. For instance, women
with:
- excessive cervical mucus, discharge or blood
- recurrent inflammatory smears
- recurrent unsatisfactory smears
In addition, the guidelines make provision for the availability of HPV testing in some specific situations. Liquid based
cytology has the practical advantage in that it offers a platform for combined cytology and HPV on one cervical cytology
specimen.
Relevant points to remember when collecting the sample
If possible avoid:
- Vaginal Medication e.g vaginal anti-fungals, spermicidals, oestrogen cream
- Douches
- Menses
- Lubricant:
- If lubricant must be used, it is advisable to use a water based product sparingly, trying to avoid the tip of the
speculum.
- Warm water may be used to lubricate and warm the speculum.
3. Liquid based cytology can be automated
While most areas of the medical laboratory have become increasingly automated, until recently cytology has remained
a labour intensive process. The LBC methodology allows for the integration of automation. This will mean a faster turnaround
of results, and the ability for the laboratory to process increased numbers of specimens.
4. Better quality slides
Most artifacts can be removed, resulting in a reduction in the number of unsatisfactory slides, meaning less recalls
for repeat smears and less uncertainty for women.
Practical implications for smear takers of LBC
The key differences for smear takers between the LBC method and the conventional Pap smear are:
- Wooden spatulas should not be used for LBC, if using a spatula it must be plastic
- Cervical cells are transferred into a vial of fixative and not smeared onto a glass slide
- Collection devices are specific for the system e.g the SurePath cervibroom must be used with the SurePath system and
not with the ThinPrep system
Cell Collection
The ideal sample consists almost entirely of squamous cells which line the ectocervix and a small number of endocervical
glandular cells to indicate that the squamocolumnar junction has been sampled.
If the cervix looks abnormal or there are abnormal symptoms the woman should be referred for colposcopic examination
irrespective of the cytology report.
Squamous cell carcinoma of the cervix accounts for 60-80% of invasive cancers of the cervix. It begins as cervical intraepithelial
neoplasia (CIN) at the squamocolumnar junction which is why it is so important to sample this site to detect early changes.
Thorough inspection of the cervix is important. This can be achieved by wiping away excess cervical mucus and using
a good light source.
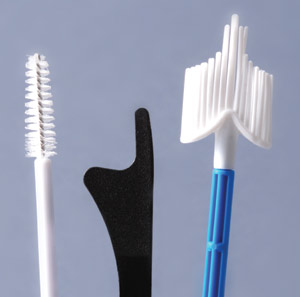 |
| Figure 1: Cervical cytology collections devices (left to right) cytobrush, spatula, cervibroom. |
The most common collection devices (Figure 1) for cervical or vault smears are a cervibroom, or a spatula and cytobrush
(preferably used in combination). Both have similar efficacy and so the choice usually depends on practitioner preference.
Many practitioners prefer the cervibroom, since only one specimen needs to be collected.
Cervibroom
This device is usually used alone. The central bristles of the broom should be inserted into the endocervical canal
deep enough to allow the shorter bristles to fully contact the ectocervix. Push gently and rotate the broom clockwise
3 to 5 times.
Spatula and Cytobrush
It is ideal to collect the spatula specimen first because of the tendency of the cytobrush to cause bleeding. The spatula
should be inserted into the cervical canal and rotated 360°.
While an adequate sample can be achieved with just a spatula, using a cytobrush increases the likelihood of obtaining
endocervical cells. Indications for using a cytobrush are:2
- repeat smears on patients with abnormalities e.g. CIN (cervical intraepithelial neoplasia), HPV
- where the anatomy of the canal has been altered by age as in post-menopausal women or by treatment such as cone biopsy
- repeating a smear where previously no endocervical cells were obtained
- abnormal bleeding
The cytobrush should be inserted into the cervical canal until the bottom bristles are only just visible. To avoid bleeding,
only rotate a ¼ to ½ turn.
SurePath and ThinPrep LBC
The two liquid based cytology systems currently used in New Zealand are SurePath (Figure 2) and ThinPrep (Figure 3).
There is no evidence of benefit of one method over the other. Both systems use different fixatives in their collection
vials and the collection devices are not interchangeable between the two systems.
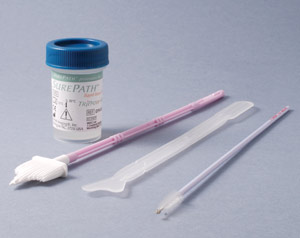 |
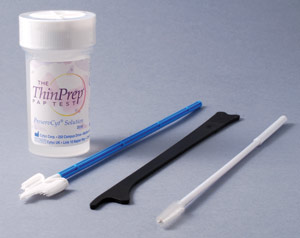 |
| Figure 2: SurePath cytology collection system |
Figure 3: ThinPrep cytology collection system |
Placing Specimen in fixative
For SurePath: The tips of the spatula and cytobrush are snapped off and placed in the fixative. The
head of the cervibroom slides off and is placed in the fixative.
For ThinPrep: The collection device is swirled in the fixative (rotating 10 times for spatula and brush,
and by pressing the brush or broom against the side of the vial), the collection device is then removed and discarded.
When using a combination of collection devices e.g. spatula with cytobrush, they should both be placed together into
the same vial of fixative (for SurePath) or rinsed in the same vial of fixative (for ThinPrep).
Advantages of LBC over conventional cytology
The advantages of this system are:
- Nearly all the cells collected are transferred into the vial of fixative from which a representative homogeneous smear
can be made.
- Most obscuring blood and inflammatory debris can be removed.
- There are no preparation artifacts such as air drying.
- Multiple slides can be prepared from one sample and part of the sample might be used for other purposes e.g. HPV testing.
- If the initial preparation is unsatisfactory, the LBC vial containing the sample can be re-examined and a second slide
may be prepared.
- It gives better quality slides leading to a decrease in the number of smears called “unsatisfactory”.
- With computer assisted screening it is expected that the average cytologist will be able to increase their throughput.
HPV testing
The National Screening Unit has confirmed that from 1st October 2009 there will be an integration of HPV testing into
the cervical screening programme guidelines. This coincides with the national adoption of LBC as the primary means of
cervical cytology, although HPV testing may not be available in all areas until funding is approved by the Ministry of
Health.
HPV is one of the most common sexually transmitted infections in the world. 15-20% of young sexually active women acquire
genital HPV infection per year. Adolescents who are sexually active have the highest rates of prevalent and incident HPV
infection rates with over 50-80% having infections within 2-3 years of initiating intercourse.3 Although HPV
infection is common, studies suggest approximately 90% of infections clear within 2 years4 with only a small
proportion progressing to cervical pre-cancer and cancer.
HPV types 16, 18, 31, 33 and 39 have a higher risk of progressing to cancer. A cervical smear showing dysplasia, intraepithelial
neoplasia or cervical cancer is almost always a result of persistent HPV infection, whereas in the absence of persistent
infection with high-risk HPV types, cervical cancer is not expected to develop.
There is good evidence that appropriately applied testing for high risk HPV types can play a useful and cost-effective
role in the management of women with abnormal cervical smears. HPV testing currently tests for 13 high risk HPV types
and has a very high negative predictive value (approx 99%).5
HPV testing can be performed at the laboratory from the same specimen as the smear (LBC), or can be performed on a separate
swab.
Using cervical cytology and HPV together
The Cervical screening guideline identifies particular areas of management of asymptomatic women with abnormal cervical
smears who may benefit from HPV testing.
This includes:
- The triage of women 30 years and over with ASCUS or low grade changes (without an abnormal smear in the last five
years).
- The follow-up of women who have been treated for a high-grade lesion.
- Post colposcopy management of women with discordant results
HPV vaccination
Gardasil was introduced to New Zealand’s vaccination programme in 2008, and offers protection against HPV types
16 and 18 (as well as 6 and 11 genital wart types).6 It is anticipated that the HPV vaccination programme
will bring about a reduction in cervical cancer rates in the future, in the meantime cervical screening is the most effective
way to reduce morbidity and mortality from cervical cancer.7
HPV is used to determine the likelihood of a low grade lesion progressing to high grade lesion for women over
30 years
There is clear agreement that women with high grade abnormalities should be referred to colposcopy but what is
less clear is how to care for women with less severe abnormalities. The complexity of managing low-grade abnormalities
relates to their mostly self-limiting nature, as well as the evidence that they harbour high grade lesions in up to 20%
of cases.8 A small number of cases of cervical cancer are diagnosed quite soon after low-grade cytology.
HPV testing in women over 30 years old will help in the management of these situations. A positive HPV test indicates
increased risk of developing a high grade lesion, and so can be a useful adjunct to the management of abnormal cell changes
seen in smears.
HPV testing is not indicated in women less than 30 years of age due to the high prevalence of HPV infection in young
women, the vast majority of which clear within 2 years and are of little clinical significance.
Follow-up of women who have been treated for a high grade lesion
Women who have been previously treated for CIN2/3 are at increased risk of further high grade disease and cervical cancer.
HPV testing changes the management of these women and may negate the need for annual smears for life for many.
Following two consecutive negative smears and HPV tests, 12 months apart, the woman will be able to return to normal
three yearly screening intervals.
When cytology and colposcopy results are inconsistent, HPV testing can help to clarify appropriate management
A single colposcopic examination can miss significant lesions. Discordant results are when a smear result differs from
the physical appearances seen at colposcopy e.g. high grade cytology with negative or satisfactory colposcopy.
In these situations HPV testing will assist in management. Following two consecutive negative smears and HPV tests,
12 months apart, the woman will be able to return to normal three yearly screening intervals.
References
- Ministry of Health, 2008. Guidelines for Cervical Screening in New Zealand, Incorporating the Management of Women
with Abnormal Cervical Smears. National Cervical Screening Programme, Wellington, New Zealand. Available from: http://www.nsu.govt.nz/Files/NCSP/NCSP_Guidelines_ALL_small(1).pdf.
Last accessed: 17 September 2009.
- Diagnostic Medlab. Cervical Cytology. Pathology Bulletin, 2007. Available from: http://www.dml.co.nz/downloads/1547_Bulletin-Cervical_Cytology2007.pdf.
Last accessed: 17 September 2009.
- Moscicki A. HPV infections in adolescents. Disease Markers. 2007; 23:229-34.
- Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of Acquisition and Clearance of Cervical Human Papillomavirus
Infection in Women from a High Risk Area for Cervical Cancer. The Journal of infectious diseases. 1999; 180:1415-23.
Available from: http://www.journals.uchicago.edu/doi/abs/10.1086/315086.
Last accessed: 17 September 2009.
- NCSP. Review of the Guidelines for Cervical Screening in New Zealand Presentation for Smeartakers, September 2008.
Available from: http://www.moh.govt.nz/moh.nsf/pagesmh/8479/$File/presentation-for-smear-takers.ppt.
Last accessed: 17 September 2009.
- BPAC. HPV Vaccines: An overview. Best Practice, Issue 12, April 2008. Available from: http://www.bpac.org.nz/magazine/2008/april/hpv.asp.
Last accessed: 17 September 2009.
- Sykes P, Sadler L, Priest P. Screening the hard to reach: improving morbidity and mortality from cervical cancer
in New Zealand. Journal of the New Zealand Medical Association. 2008; 121:1277.
- Al-Nafussi A, Hughes D. Histological features of CIN3 and their value in predicting invasive microinvasive squamous
carcinoma. British Medical Journal. 1994; 47:799-804.