Module 2: Early Medical Abortion
3. Medicines used for EMA
For a video discussing EMA and the medicines used, click here.
Mifepristone
Mifepristone (e.g. Mifegyne®) is a synthetic steroid (Figure 1) with anti-progestational properties as a result of competition with progesterone for progesterone receptors. In people at doses of ≥ 1 mg/kg, mifepristone antagonises the endometrial and myometrial effects of progesterone. During pregnancy it sensitises the myometrium to the contraction-inducing action of prostaglandin. The recommended dose in the datasheet is 200 mg to 600 mg depending on gestation. As peer reviewed research has shown no benefit from the use of the higher 600 mg dose, the dose prescribed in clinical practice is 200 mg.
- Absorption: bioavailability ~69%, time to peak concentration is approximately 90 minutes
- Half-life: 20 – 85 hours
- Storage: No special storage conditions are required.
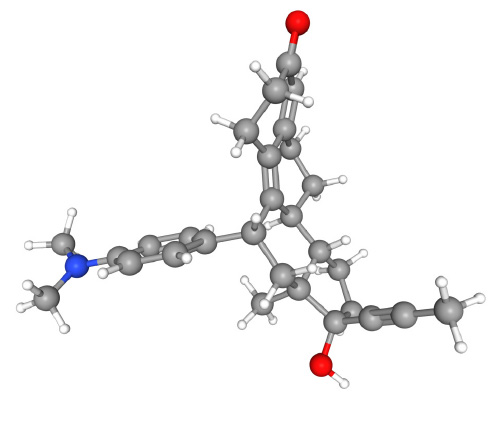
Figure 1. Mifepristone (C29H35NO2, IUPAC name (8S,11R,13S,14S,17S)-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one). PubChem Identifier: CID 55245.
Misoprostol
Misoprostol (e.g. Cytotec®) is a synthetic prostaglandin E1 analogue (Figure 2), which induces contractions of the smooth muscle fibres in the myometrium and relaxation of the cervix. Oral and sublingual/buccal routes have the advantage of rapid onset of action, while the vaginal application results in prolonged activity and greater bioavailability. Each tablet is 200 micrograms.
- Absorption: 9 – 15 minutes after sublingual, buccal, oral or vaginal application
- Half-life: 20 – 40 minutes
- Storage:
- Blister packs – Store at or below 25°C and protect from moisture
- Bottles – Store at or below 30°C and protect from moisture
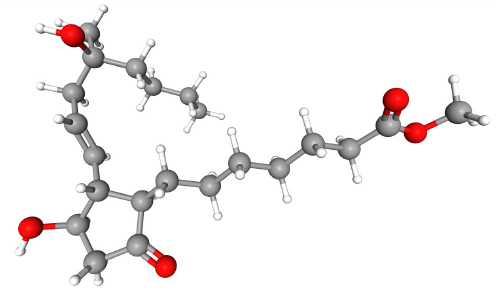
Figure 2. Misoprostol (C22H38O5, IUPAC name methyl 7-[(1R,2R,3R)-3-hydroxy-2-[(E)-4-hydroxy-4-methyloct-1-enyl]-5-oxocyclopentyl]heptanoate).
PubChem Identifier: CID 5282381.
Teratogenicity
The Medsafe datasheet for Mifegyne® (mifepristone) states that malformations with sub-abortive test doses have been observed in rabbits, but not in rats, mice or monkeys, and that “the data is too limited to determine whether the drug is a human teratogen”. Rare congenital malformations, including limb defects, club foot, central nervous system (CNS) and palate anomalies have been reported with the therapeutic use of misoprostol in clinical practice but no clear causal relationship has been established. The teratogenic mechanism appears to be related to the induction of uterine contractions that deform the embryo, resulting in vascular disruption, haemorrhage and cell death. The Medsafe datasheet for Cytotec® (misoprostol) states “Möbius sequence (i.e. palsies of cranial nerves VI and VII) and terminal transverse limb defects have been associated with first trimester exposure to misoprostol. Other defects including arthrogryposis have been observed. Misoprostol is contraindicated in women who are pregnant.”
People seeking an abortion need to be informed of the risk of failure of EMA and the potential teratogenic risk to the fetus. Follow-up pregnancy testing is offered to confirm the abortion is complete. If a viable ongoing pregnancy is diagnosed at follow-up, it is recommended that the patient is advised to complete the abortion. A second EMA process, or an aspiration abortion, should then be performed with the patient’s consent. If the patient decides to continue with their pregnancy after a failed EMA, careful ultrasound monitoring of the pregnancy, with a special attention to the limbs, is recommended.
Registered nurse prescribing
The Te Kaunihera Tapuhi o Aotearoa Nursing Council of New Zealand “Guidance for registered nurse prescribing in primary health and specialty teams” (March 2022) contains a medicines list which includes both mifepristone and misoprostol for use in obstetrics and medical abortion.
Use of approved medicines for unapproved indications
When a medicine is registered with Medsafe, it is subject to specific approval parameters, including indications, contraindications, cautions, doses, routes of administration and formulations. If a medicine is prescribed outside of these parameters, this is considered to be an unapproved use of the medicine (also known as “off-label” use). However, under Section 25 of the Medicines Act 1981 authorised prescribers working within the scope of their practice can still procure the supply of any medicine for use in patients under their care, provided requirements under the Code of Health and Disability Services Consumers’ Rights 1996 have been applied. People have the right to be fully informed about unapproved medicines and any safety concerns, including in writing (if requested), prior to consenting to their use for EMA. Verbal informed consent from the patient is sufficient and this should be documented in the patient notes. It is not necessary to obtain written consent.
Mifepristone (Mifegyne®) is currently licensed for medical abortion up to 63 days of amenorrhoea. However, the New Zealand Aotearoa Abortion Clinical Guideline recommends that an EMA can be provided for pregnancies up to 10 weeks. The approved mifepristone dose for <50 days of amenorrhoea is 600 mg (3 tablets of 200 mg each) and between 50–63 days of amenorrhoea it is 200 mg. The New Zealand Aotearoa Abortion Clinical Guideline recommends 200 mg at all gestations. Therefore, the 200 mg regimen is Unapproved use prior to 50 days and between 63 and 70 days.
Misoprostol (Cytotec®) is currently approved for treatment of gastric and duodenal ulcers, and unapproved for all obstetric indications, including abortion.
Anti-D prophylaxis
As Anti-D prophylaxis is not recommended for use for EMA, a group and antibody screen is not required prior to an EMA and should not delay care.
