Initiating pharmacological treatment for excessive respiratory secretions
There is limited evidence supporting pharmacological interventions for excess respiratory secretions;2 they do not remove secretions already present,7 and only reduce the amount of new secretions forming. Therefore, the benefits of pharmacological options need to be weighed against the adverse effects, and in most cases will often only be appropriate if the patient is unconscious (or semi-conscious).
Hyoscine butylbromide (Buscopan; unapproved indication) is the first-line treatment for patients with excessive respiratory secretions in the last days of life.5 It reduces secretions due to its anticholinergic action on smooth muscle,5 however, this mechanism can also cause adverse effects, e.g. dry mouth, constipation and urinary retention.8 Therefore, patients should be regularly evaluated while receiving treatment, and have mouth care prioritised, even if unconscious.
Give 20 mg, subcutaneously, every two to four hours, as needed (maximum 120 mg daily).5 Ideally, patients should be reviewed within six hours,4 although it may take up to 12 hours to see a therapeutic effect.2 Hyoscine butylbromide should be stopped if the patient shows no improvement after 12 hours.2 Patients who do show benefit can be switched to a continuous subcutaneous infusion of 40 – 80 mg, hyoscine butylbromide over a 24-hour period.4
N.B. Hyoscine hydrobromide (Scopoderm TTS) patches are usually avoided in this setting due to a high risk of severe adverse effects, e.g. delirium.9
Glycopyrronium bromide is another anticholinergic medicine used to decrease salivary and respiratory secretions.9 It is an alternative treatment for excessive respiratory secretions in patients in the last days of life (unapproved indication) if hyoscine butylbromide is not available or appropriate.8 Glycopyrronium bromide can be given as 200 micrograms, subcutaneously, every four hours, as needed.9 A continuous subcutaneous infusion of 0.6 – 1.2 mg over a 24-hour period may also be considered in patients who show improvement in symptoms.9
 Practice Point: Adverse effects of anticholinergic medicines need to be considered in the context of the patient’s clinical condition and the goals of treatment; some cautions and contraindications for these medicines may not apply to patients in the last days of life, e.g. bowel obstruction (or paralytic ileus), risk of glaucoma, hypertension, tachycardia. Discussion with the local hospice or palliative care team may help with decision making. 8
Practice Point: Adverse effects of anticholinergic medicines need to be considered in the context of the patient’s clinical condition and the goals of treatment; some cautions and contraindications for these medicines may not apply to patients in the last days of life, e.g. bowel obstruction (or paralytic ileus), risk of glaucoma, hypertension, tachycardia. Discussion with the local hospice or palliative care team may help with decision making. 8
N.B. Do not prescribe hyoscine butylbromide and metoclopramide together as they have opposing clinical effects.8
Regularly review the patient’s ongoing care plan and ensure that interventions are current and still appropriate for the clinical condition of the patient.2 In some situations, limiting or stopping hydration may be considered to reduce the impact of pulmonary oedema or respiratory secretions.
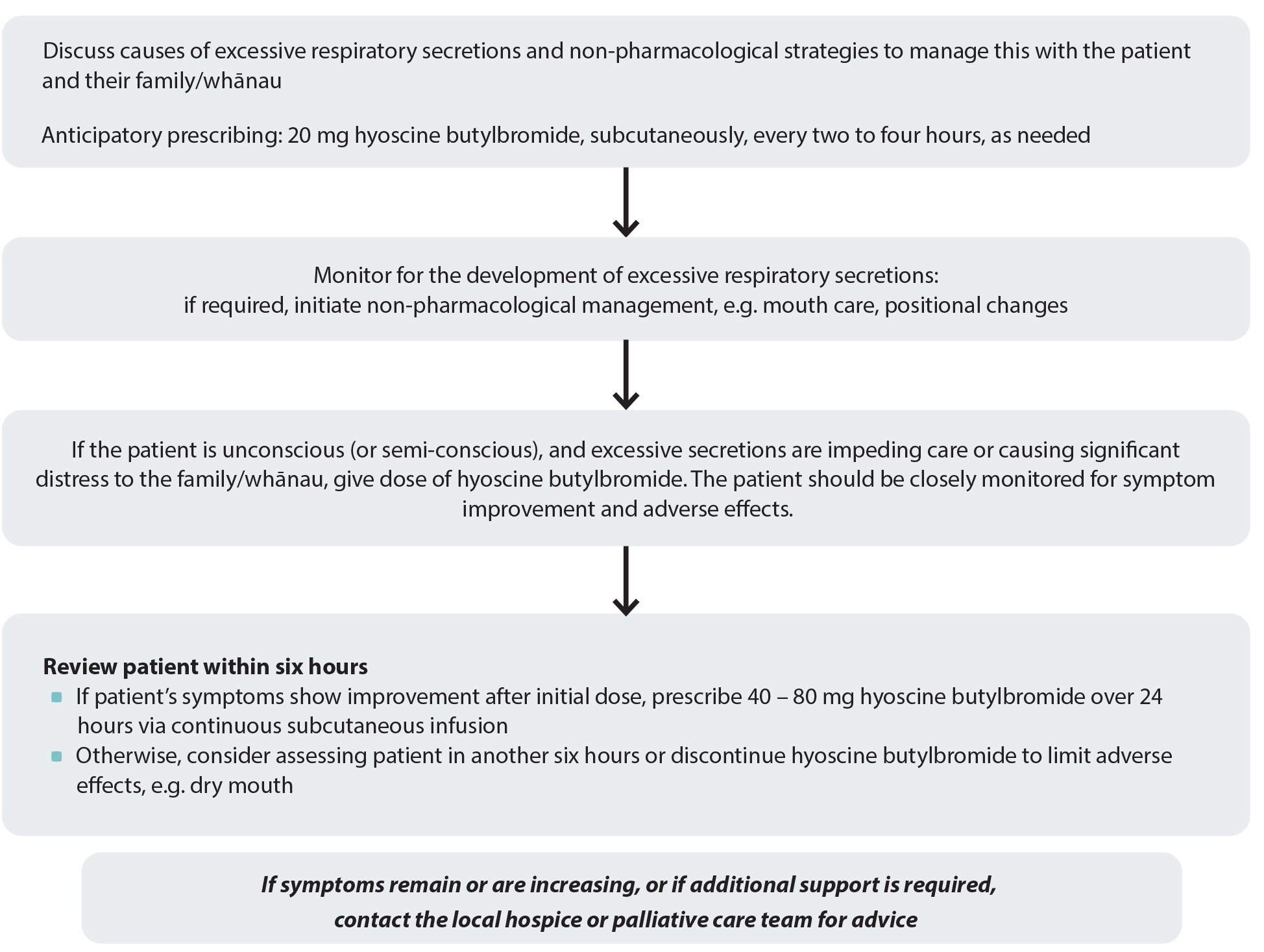
Figure 1. Anticipatory prescribing for patients with excessive respiratory secretions. Adapted from South Island Palliative Care Workstream, 2020.4
Acknowledgement
Thank you to the following experts for review of this article:
- Dr Kate Grundy, Palliative Medicine Physician, Clinical
Director of Palliative Care, Christchurch Hospital Palliative
Care Service and Clinical Lecturer, Christchurch School of
Medicine
- Vicki Telford, Clinical Nurse Specialist, Nurse Maude
Hospice Palliative Care Service, Christchurch
- Dr Helen Atkinson, General Practitioner and Medical
Officer, Harbour Hospice
- Dr Robert Odlin, General Practitioner, Orewa Medical
Centre
- Fraser Watson, Extended Care Paramedic Clinical Lead,
Hato Hone St John
N.B. Expert reviewers do not write the articles and are not responsible for the final content.
bpacnz retains editorial oversight of all content.
This resource is the subject of copyright which is owned by bpacnz.
You may access it, but you may not reproduce it or any part of it except in the limited situations described in the
terms of use on our website.
Article supported by Te Aho o Te Kahu, Cancer Control Agency.

References
- Crawford GB, Dzierżanowski T, Hauser K, et al. Care of the adult cancer patient at the end of life: ESMO Clinical Practice Guidelines. ESMO Open 2021;6:100225. https://doi.org/10.1016/j.esmoop.2021.100225
- National Institute for Health and Care Excellence (NICE). Care of dying adults in the last days of life. 2015. Available from: https://www.nice.org.uk/guidance/ng31 (Accessed September, 2023).
- Ministry of Health – Manatū Hauora. Te Ara Whakapiri Toolkit: care in the last days of life. 2017. Available from: https://www.health.govt.nz/system/files/documents/publications/te-ara-whakapiri-toolkit-apr17.pdf (Accessed September, 2023).
- South Island Palliative Care Workstream. Te Ara Whakapiri Symptom management in the last days of life. 2020. Available from: https://www.sialliance.health.nz/wp-content/uploads/Symptom-management-in-the-last-days-of-life_SIAPO.pdf (Accessed September, 2023).
- Azhar A, Hui D. Management of physical symptoms in patients with advanced cancer during the last weeks and days of life. Cancer Res Treat 2022;54:661–70. https://doi.org/10.4143/crt.2022.143
- PDQ Supportive and Palliative Care Editorial Board. Last days of life (PDQ®): health professional version. In: PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US) 2007 (updated 2023). Available from: http://www.ncbi.nlm.nih.gov/books/NBK65868/ (Accessed September, 2023).
- Chapman L, Ellershaw J. Care in the last hours and days of life. Medicine 2020;48:52–6. https://doi.org/10.1016/j.mpmed.2019.10.006
- MacLeod R, Macfarlane S. The palliative care handbook. 9th ed. 2019. Available from: https://www.hospice.org.nz/wp-content/uploads/2019/03/Palliative-Care-Handbook.pdf (Accessed September, 2023).
- New Zealand Formulary (NZF). NZF v135. Available from: www.nzf.org.nz (Accessed September, 2023).
