Published: 17 July 2020 | Updated: 15 April 2024 | What's changed?
15 April 2024 Benralizumab is now funded with Special Authority approval for patients with severe eosinophilic asthma
Key practice points:
- Budesonide/formoterol is now the preferred treatment for all adolescents* and adults with symptoms of asthma; this
can be used both for maintenance treatment and for relief of symptoms as needed, i.e. only one inhaler is
required
- An inhaled corticosteroid (ICS) should be prescribed to all patients with asthma; those not taking budesonide/formoterol
need an ICS or a combination ICS/long-acting beta2-agonist (LABA) as an alternative with a short-acting beta2-agonist
(SABA), i.e. two inhalers are required
- SABAs are harmful when taken regularly without concurrent ICS treatment; reiterate the importance of ICS adherence
to all patients taking a SABA or recommend switching treatment to budesonide/formoterol
- Formoterol is the only LABA that can be used for immediate symptom relief; ICS/LABA combinations that do not contain
formoterol should not be used for acute reliever treatment
- The biological medicines omalizumab, mepolizumab and benralizumab are funded with Special Authority approval for people with
severe asthma who meet certain criteria, following application in secondary care
* Defined as people aged 12–17 years
N.B. Further information on aspects of care not covered in this article, e.g. asthma assessment and education, treatable traits, and
environmental and behavioural factors affecting asthma control are available from:
“Managing adults with asthma in primary care: the four-stage consultation”
For more than 50 years, people with asthma have been prescribed a short-acting beta2-agonist (SABA) as
the first-line treatment for symptom relief.1 The accumulated weight of evidence has, however, shifted and
the new Asthma and Respiratory Foundation NZ guidelines (2020) prefer budesonide/formoterol over SABAs for the relief
of asthma symptoms. This change has resulted in the term “anti-inflammatory reliever” (AIR) therapy to describe treatment
with a sole inhaler combination of the inhaled corticosteroid (ICS) budesonide and the fast-onset, long-acting beta2-agonist
(LABA) formoterol (see: “AIR includes SMART”).2
AIR includes SMART
Budesonide/formoterol is also the basis of the Single ICS/LABA Maintenance And Reliever Therapy (SMART) that has existed for more than 15 years.
The term AIR includes SMART, the use of budesonide/formoterol for both maintenance and reliever treatment, and the use of budesonide/formoterol
only for immediate symptom relief (as needed).
A stepwise pathway for asthma management
Asthma treatment is stepped up or down in order to determine the optimal level that controls symptoms, manages exacerbation
risk and minimises adverse effects.2 The level of treatment required to achieve control is in turn used to
define asthma severity, e.g. mild asthma is well-controlled at the first step of treatment and severe asthma is uncontrolled
despite maximal treatment taken correctly.3
An ICS is recommended for all adolescents and adults with asthma
The new guidelines provide clearer recommendations on when ICS treatment should be initiated, in the form of two treatment
pathways. All adolescents* and adults with asthma, including those with exercise-induced or infrequent symptoms, should
now use an ICS-containing inhaler to control their symptoms and reduce their risk of exacerbations. This provides consistent
messaging to patients and prevents them developing a reliance on a SABA that can be difficult to overcome later. Budesonide/formoterol
is preferred (Figure 1, AIR), but an ICS with as needed SABA is an alternative for patients not switched to AIR (Figure
2).2, 3
While many primary care clinicians have been recommending early ICS initiation for years, this new recommendation will
provide clarity in the management of patients with infrequent or exercise-associated symptoms as well as aiding adherence
in those who were not regularly taking ICS treatment.
* Defined as people aged 12–17 years
New asthma guidelines are standardising care
By providing precise guidance on when an ICS should be initiated and widely recommending the use of budesonide/formoterol,
the new guidelines are standardising asthma management.2 These recommendations are consistent with the Global
Initiative for Asthma (GINA) 2020 guidelines.3 It is hoped that by optimising and standardising care, patient
outcomes will be improved which will help to reduce asthma inequities experienced by some of New Zealand’s most vulnerable
communities (see: “Asthma-related health inequities in New Zealand”).
Asthma-related health inequities in New Zealand
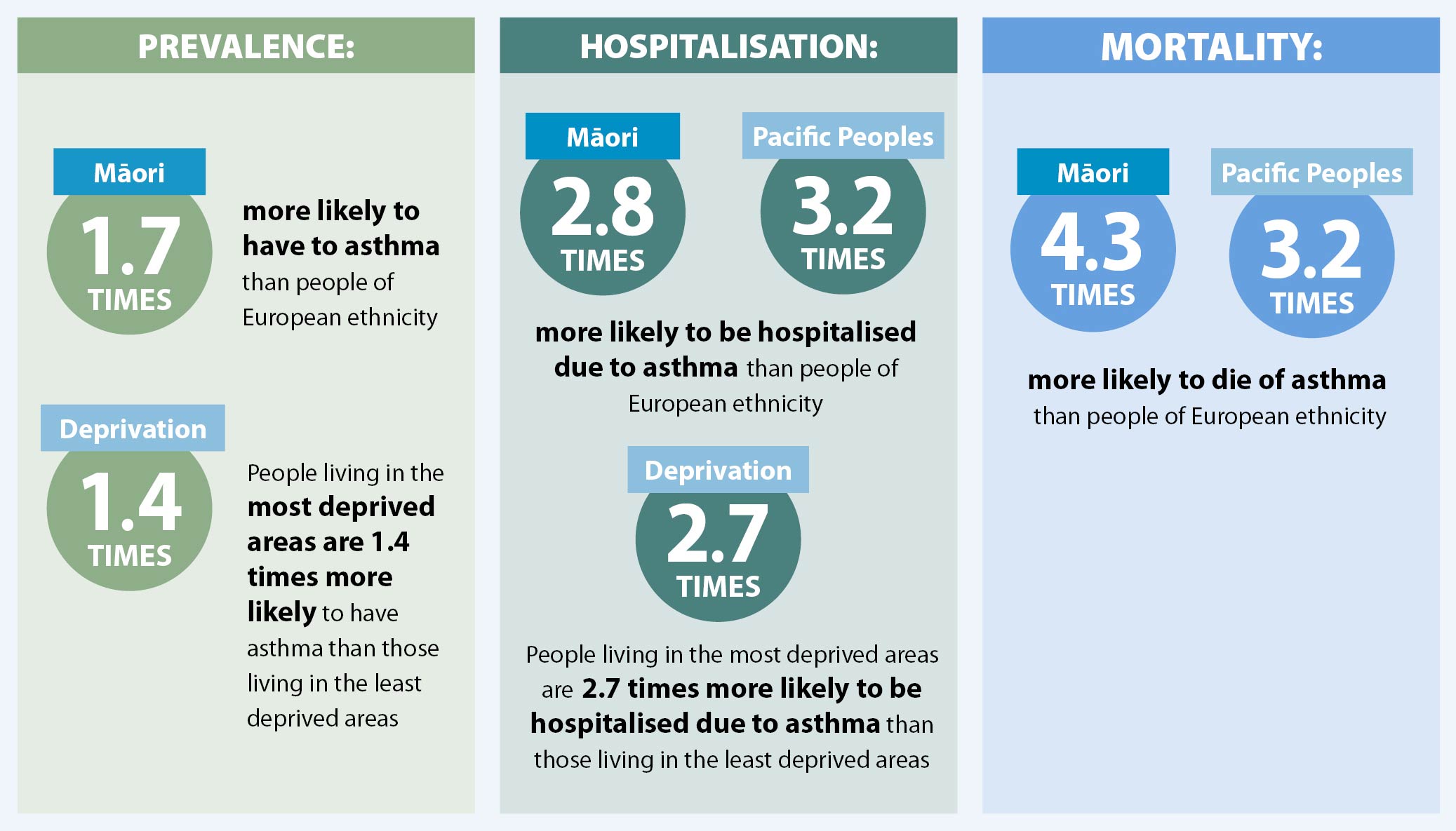
Asthma is highly prevalent in New Zealand with 11.5% of people aged over 15 years diagnosed and receiving treatment
to control their symptoms.4 Māori and Pacific peoples and those living in deprived areas are more likely
to have asthma and more likely to be severely affected by it (see below).4, 5 Factors that contribute to
an increased risk of asthma-related events include:2
- Damp, cold, or mouldy housing*
- Overcrowded housing†
- Higher rates of smoking
- Lower incomes and the cost of transport, consultations and prescriptions
- Reduced access to healthcare and a lack of continuity of care
- Suboptimal management
- Reduced health literacy
- Occupational asthma
* Referral to local housing services, e.g. for insulation, curtains, housing assistance, may be appropriate for eligible
families. Links to national and local healthy homes and heating support services are available on HealthPathways:
www.healthpathwayscommunity.org
Ceiling and underfloor insulation is now compulsory in all rental homes where it is practicable to install:
www.tenancy.govt.nz/maintenance-and-inspections/insulation/compulsory-insulation
† Organisations such as Habitat for Humanity may be able to provide help to families unable to afford home ownership
or those struggling to maintain their home: https://habitat.org.nz/what-we-do
Institutional racism in health care is an issue for asthma management that needs to be addressed. Māori are less likely
than New Zealand Europeans to be prescribed an ICS, to have an asthma action plan or to receive appropriate asthma education.2 AIR
therapy with budesonide/formoterol, either alone or as maintenance and reliever treatment, i.e. SMART, has been shown
to improve asthma control in Māori patients.6, 7
Challenge yourself to consider your approach to asthma management and whether there are changes that you could make
to help ensure equitable outcomes for Māori. For example:
- Investing more time in asthma education for disadvantaged patients and ensuring they understand the benefits of taking
regular ICS
- Actively recalling Māori patients with asthma to recommend switching to AIR therapy
- Considering what your practice can do to reduce barriers to consultations and access to medicines
- Referring people more consistently to support services and, if required, to secondary care
Pacific peoples and people with disadvantaged backgrounds experience inequities and a significant burden of respiratory
disease too; similar considerations are required to improve health outcomes for this group.
AIR therapy is the use of budesonide/formoterol in two ways:2
- Budesonide/formoterol as needed only for symptom relief, i.e. without maintenance treatment (Step 1)
- Budesonide/formoterol for maintenance treatment with an additional as needed dose for symptom relief (Step 2 and
3); also referred to as Single combination ICS/LABA inhaler Maintenance And Reliever Therapy (SMART)
Reassure patients that budesonide/formoterol is as effective as a SABA for symptom relief,3 including
during an exacerbation if the patient is able to use the turbuhaler device (the recommended delivery method). All patients
taking SABA-only treatment should be switched to budesonide/formoterol. Patients who are using an ICS with as needed SABA
can also be encouraged to switch, especially if they do not have good asthma control. Budesonide/formoterol should not
be used for symptom relief if the patient is taking a different ICS/LABA for maintenance treatment, i.e. two LABAs should
not be combined.3
Formoterol is the only LABA that can be used for immediate symptom relief* because it
has a relatively rapid onset. Fluticasone/salmeterol (Seretide, Rexair [see: “Funding changes
for fluticasone and fluticasone with salmeterol inhalers”]) and fluticasone/vilanterol (Breo Ellipta) have not been
studied as reliever treatments alone and these medicines are not appropriate for immediate symptom relief; patients
using these inhalers require a SABA. Inhaled formoterol produces bronchodilation after one to three minutes, whereas
inhaled salmeterol has an onset of 10 to 20 minutes, with the full effect of salmeterol taking several hours.8,
9 Patients who take fluticasone/vilanterol generally experience an improvement in lung function within 15 minutes,10 but
this is not usually rapid enough for acute relief.
* Beclomethasone/formoterol may also be suitable as an AIR, however, this medicine combination is not available in New
Zealand.3
There are two types of funded budesonide/formoterol inhalers in New Zealand, however, only the Symbicort
Turbuhaler dry powdered inhalers (DPI – 200/6 and 100/6) are approved for reliever therapy.2 The Vannair
metered dose inhalers (MDI – 200/6 and 100/6) may, however, be preferred by patients who find the Turbuhaler difficult
to manipulate; prescribing this inhaler as an unapproved indication may be reasonable if it is likely to result in improved
adherence. The Symbicort Turbuhaler DPI, 400/12 micrograms inhaler should not be used as a reliever.2
One actuation of the 200/6 (preferred) or 100/6 formulation of budesonide/formoterol is taken as needed
for symptom relief, in place of two SABA doses.2
The maximum dose of budesonide/formoterol (200/6 or 100/6) is 12 actuations a day, i.e. 72 micrograms of
formoterol.2, 3
How AIR therapy works
The 200/6 combination Symbicort Turbuhaler is preferred for AIR therapy and the dosing recommendations below (see Figure
1 and Table 1) are based on this inhaler.2 Additional inhalers are not required as the budesonide/formoterol
Turbuhaler is used for both maintenance treatment and symptom relief.
Step 1 is the use of budesonide/formoterol, one actuation at a time, for symptom relief only.2 Most
patients with asthma can begin on Step 1, i.e. those initially presenting with symptoms ranging in frequency from occasional
to multiple times a month, but not daily.3 Patients who have problems adhering to inhaled medicines are likely
to prefer this step which is the minimum treatment recommended for all adolescents and adults with asthma.2
Step 2 is the use of budesonide/formoterol as maintenance treatment, either one actuation, twice daily,
or two actuations, once daily, with additional doses of budesonide/formoterol, one actuation at a time, as needed to relieve
symptoms.2 This may be appropriate for patients who initially present with symptoms most days or waking with
asthma once a week or more.3
Step 3 is the use of budesonide/formoterol as maintenance treatment at a higher dose, two actuations, twice
daily, with budesonide/formoterol, one actuation at a time, as needed to relieve symptoms.2 This level of
treatment is unlikely to be necessary for most patients newly diagnosed with asthma but may be required as step up treatment
if symptoms are uncontrolled at Step 2.
Patients should move up or down steps, depending on their level of control, i.e. patients using the 200/6
Symbicort Turbuhaler:11
- Step down, if using 0 –2 reliever actuations per week
- Continue on their current step, if using 3 – 7 reliever actuations per week
- Step up, if using > 7 reliever actuations per week
If the patient’s symptoms are uncontrolled at Step 3 consider “add-on” treatments (see: “Additional
asthma medicines “) and review by a respiratory physician.2
Switching patients already on asthma treatment
Patients can be switched immediately to AIR therapy from using an as needed SABA:11
- Step 1 of AIR is appropriate for patients with good control previously taking a SABA alone or an ICS with as needed
SABA
- Step 2 of AIR is appropriate for patients with good control previously taking an ICS/LABA at a standard dose with
as needed SABA
- Step 3 of AIR is appropriate for patients previously taking an ICS/LABA at a higher than standard dose with as needed
SABA
If the switch to AIR is made following a severe exacerbation, consider stepping treatment up when the switch is made
to reduce the patient’s exacerbation risk, once adherence and technique have been assessed. When the patient’s condition
is stable, treatment may be able to be stepped down.
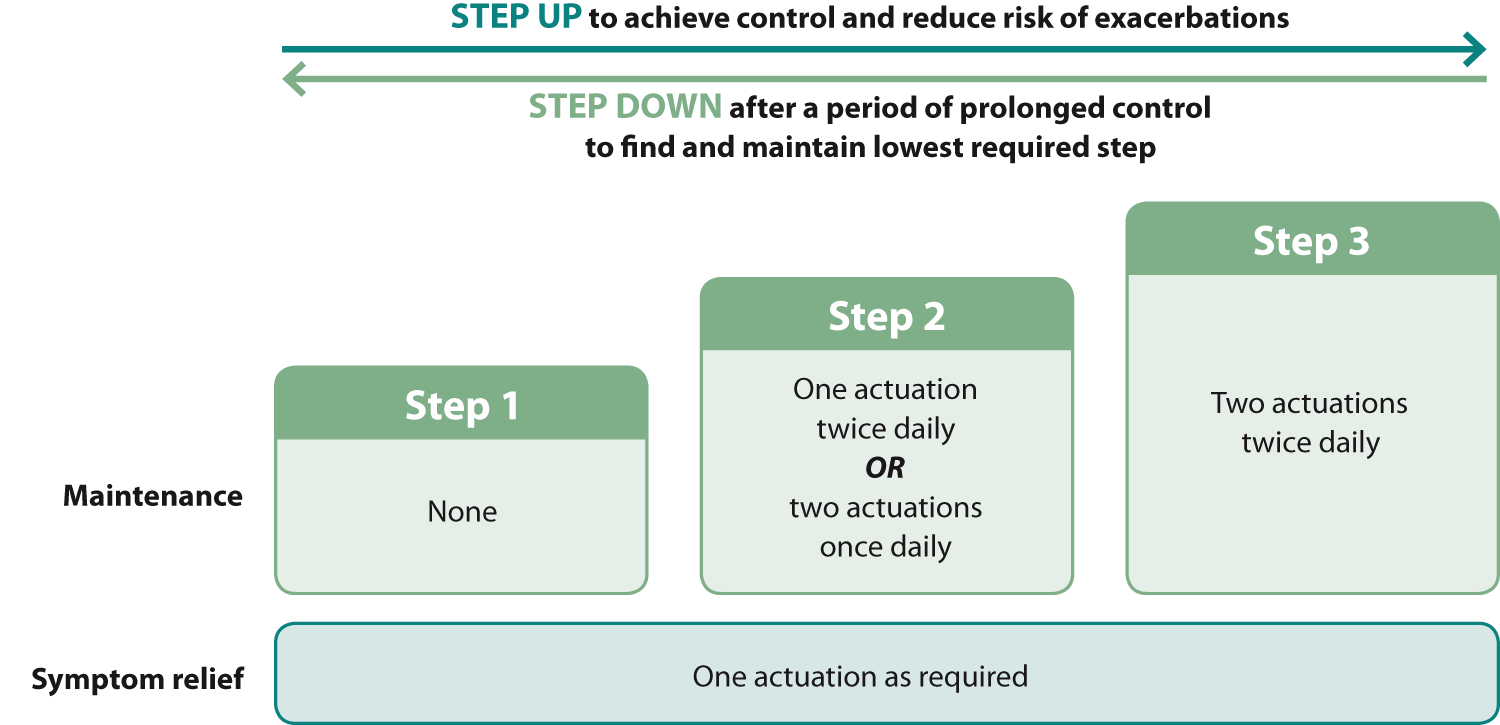
Figure 1: AIR therapy using budesonide/formoterol (200 micrograms + 6 micrograms preferred), adapted from Beasley et al (2020)2
Table 1: The recommended (standard) daily dose for initiation of ICS and ICS/LABA treatment in adolescents and adults with asthma2
| Inhaled corticosteroid (ICS) |
Total daily dose (micrograms) |
| Beclomethasone dipropionate |
400 – 500 |
| Beclomethasone dipropionate extrafine |
200 |
| Budesonide |
400 |
| Fluticasone propionate |
200 – 250 |
| Fluticasone furoate |
100 |
Key things to remember with AIR therapy:2
- Patients should not be prescribed a SABA while using AIR therapy
- Only one actuation of the budesonide/formoterol 200/6 or 100/6 formulations is required for acute symptom relief (as
compared to two actuations of a SABA)
- High-dose budesonide/formoterol (400 micrograms/12 micrograms) is not appropriate for AIR therapy
- Health professionals should continue to use a SABA to manage patients with severe acute asthma exacerbations in the
clinic; there is insufficient evidence to recommend using budesonide/formoterol in this setting
The evidence behind the new recommendations
Budesonide/formoterol is preferred over a SABA for the immediate relief of asthma symptoms for four main reasons:
- People with infrequent symptoms are often non-adherent to ICS treatment which increases their risk of SABA-related
adverse effects due to SABA overuse.3
- Compared to SABA-only treatment, as needed budesonide/formoterol results in at least 60% fewer severe exacerbations
in patients with mild asthma.12–14
- Compared to ICS treatment with as needed SABA, as needed budesonide/formoterol significantly reduces the frequency
of severe exacerbations in patients with mild asthma, and is associated with a lower daily average ICS dose.3, 7, 14
- Compared to other ICS/LABAs with as needed SABA in patients with a history of exacerbations, budesonide/formoterol
for maintenance and as needed treatment significantly reduces exacerbation risk.3
Early ICS treatment is associated with improvements in lung function and outcomes. People with asthma who
start an ICS early have a greater response to treatment than those where initiation is delayed.3 For example,
one study showed a 22% increase in lung function following ICS initiation in patients who had asthma symptoms for less
than six months.15 This compared to a 6% increase in lung function following ICS initiation after two to
five years of symptoms and a 2% increase in function in patients with symptoms for more than ten years.15 Furthermore,
severe asthma exacerbations are associated with an increased rate of lung function decline that may be reduced if the
patient is taking an ICS.16 Finally, the risk of serious asthma-related events is substantially reduced by
the regular use of an ICS; most asthma-related hospitalisations and deaths can be prevented with this intervention.17
The new guideline provides a second treatment pathway for patients who are not switched to AIR treatment. This alternative
is an ICS or ICS/LABA taken every day with SABA as needed for symptom relief (Figure 2).2 This option is
only appropriate for patients who are likely to be adherent to ICS treatment.3 Patients with good asthma
control using an ICS or ICS/LABA and a SABA taken as needed may wish to remain on their current treatment regimen and
this is a reasonable approach.
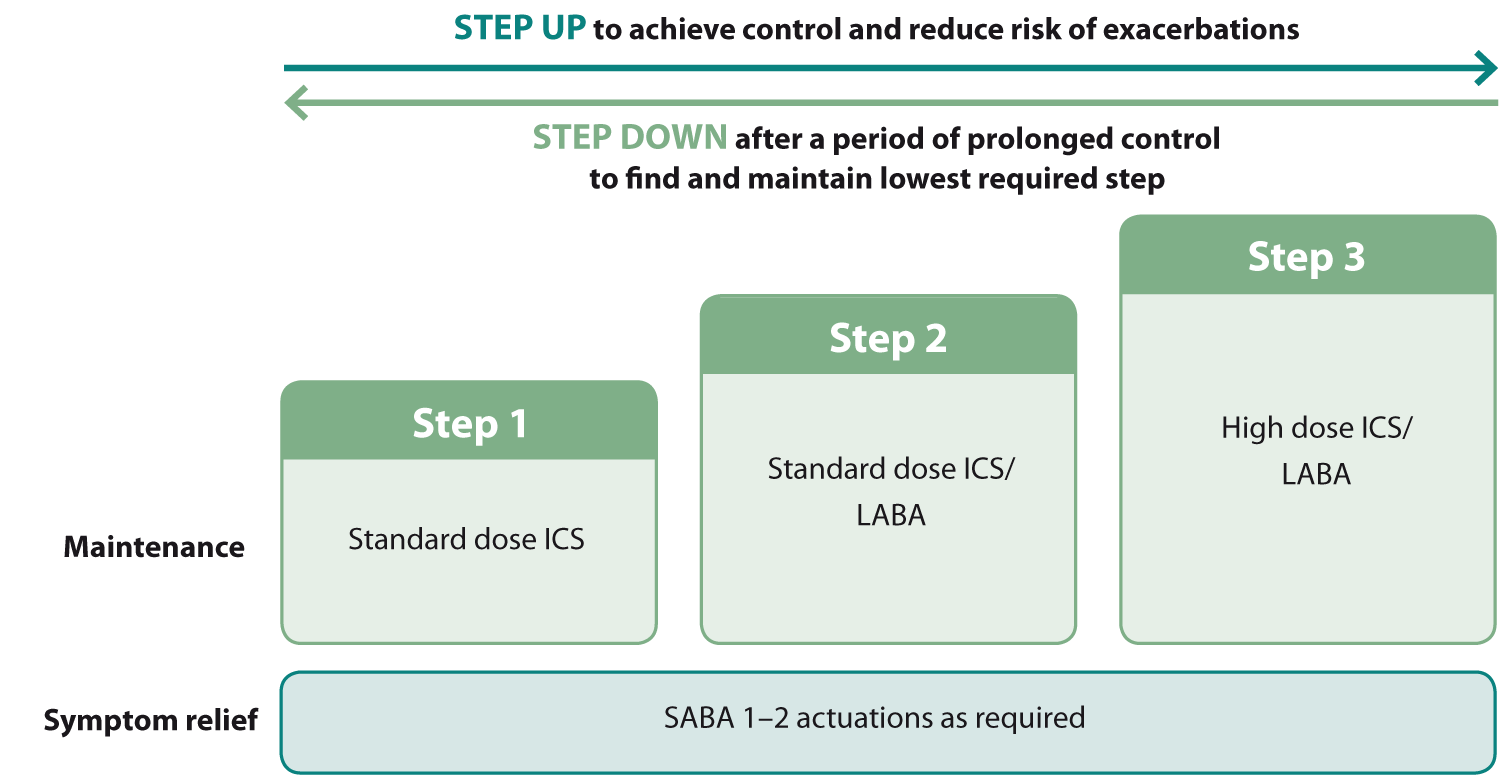
Figure 2: Alternative asthma treatment with ICS and as needed SABA for symptom relief, adapted from Beasley et al (2020)2
The stepwise use of ICS with SABA has changed
All patients previously prescribed a SABA only, now need to switch to AIR therapy or be advised to take another ICS
containing inhaler every day, including those with mild asthma or exercise-associated symptoms.2 Previously,
Step 1 was SABA only treatment without an ICS, which is no longer recommended in the long-term management of asthma.2,
18
This change occurred because SABA use without an ICS is associated with a higher exacerbation risk as it increases inflammation
and sensitivity to allergens.3, 12 Furthermore, regular SABA use causes tachyphylaxis, thereby creating a
feedback loop where patients need a SABA more often, but the medicine becomes less effective the more it is needed.12 The
use of three or more SABA cannisters a year is associated with an increased risk of emergency department visits or hospitalisation,
independent of asthma severity.3
Health professionals should, however, continue to use a SABA via a spacer to manage patients with severe acute asthma
exacerbations in the clinic.
ICS with as needed SABA therapy
Step 1 is standard dose beclomethasone, budesonide or fluticasone (Table 1) with as needed use of a SABA
for symptom relief. Most patients with asthma can begin on Step 1, i.e. those presenting initially with symptoms ranging
in frequency from occasional to multiple times a month, but not daily.3
Step 2 is standard dose fluticasone propionate/salmeterol or fluticasone furoate/vilanterol with as needed
use of a SABA.2 This may be appropriate for patients presenting initially with troublesome symptoms on most
days, or who wake due to asthma once a week or more.3 LABAs should not be prescribed in separate inhalers
to an ICS as LABA monotherapy in patients with asthma is associated with a small but significantly increased mortality
risk.19
Step 3 is fluticasone propionate/salmeterol or fluticasone furoate/vilanterol at ICS doses higher than
standard (Table 1) with as needed use of a SABA.2 This level of treatment is unlikely to be necessary for
most patients newly diagnosed with asthma but may be required as step up treatment for those established on treatment
if symptoms are uncontrolled at Step 2.
If the patient’s symptoms are uncontrolled at Step 3 further discussion about switching to AIR may be appropriate or
consider “add-on” treatments (see: “Additional asthma medicines“) and review by a respiratory physician.2
N.B. technically budesonide/formoterol is also an ICS/LABA option at Steps 2 and 3, however, if a patient was using
this, it would be illogical to not use it as part of an AIR therapy regimen.
Discussing ICS use with patients

It can be helpful to remind patients that even if they have infrequent or intermittent symptoms, their airways are likely
to have some degree of chronic inflammation and that use of an ICS is the most effective way of controlling this (see:
“The evidence behind the new recommendations”).12, 20
Some patients may have concerns about the use of ICS treatment, e.g. adverse effects or “steroid stigma”; they may be
reassured that systemic adverse effects with ICS taken at standard doses are very uncommon.
Women who are planning to conceive or who are pregnant should continue taking any inhaled asthma medicines as normal
during pregnancy.2 The theoretical risk associated with asthma medicines during pregnancy is clinically insignificant
compared to the risk for both the mother and the baby of stopping treatment.2
N.B. All people who are prescribed an ICS to control asthma are eligible for a funded influenza vaccination, regardless of treatment adherence.
Considerations before stepping up treatment
Before considering a change to the patient’s treatment for either AIR therapy or ICS with as needed SABA, the following
should be checked:
- Assess inhaler technique and discuss adherence; the most common reasons for poor asthma control
- Consider if the diagnosis is correct and if other factors are contributing:
- Overlapping disorders, e.g. chronic obstructive pulmonary disease, bronchiectasis, nasal polyposis or rhinitis
- Co-morbidities, e.g. obesity, gastro-oesophageal reflux disease, anxiety or obstructive sleep apnoea
- Environmental factors, e.g. tobacco smoke or cold, damp housing
- Medicines, e.g. aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) or beta-blockers
- Confirm the patient’s understanding of their management plan
- Encourage non-pharmacological interventions as appropriate, e.g. smoking cessation, weight loss, regular exercise
and breathing exercises
- Discuss any further barriers to self-care, e.g. access to medicines or additional spacer requirements
Further information on smoking cessation is available from:
https://bpac.org.nz/BPJ/2015/October/smoking.aspx
Asthma action plans, including plans written in te reo Māori and Samoan, are
available from: www.asthmafoundation.org.nz/resources
Further information on breathing exercises is available from:
www.asthmafoundation.org.nz/resources/what-is-buteyko
“Add-on” medicines for asthma are not routinely recommended, but they may be appropriate for patients with:
- Mild symptoms who are unable to tolerate an ICS
- Uncontrolled or severe asthma, following review by a respiratory physician
Mild asthma: add-on treatments
Montelukast, an oral leukotriene receptor antagonist, may be appropriate as an additional treatment for
all patients with aspirin-exacerbated asthma and potentially others with symptoms not controlled with standard treatments.2 Montelukast
is less effective at controlling symptoms, and particularly at reducing exacerbation risk, than ICS treatment.3 Montelukast
has been associated with the development of neuropsychiatric adverse effects, including abnormal dreaming, insomnia and
aggression.21
Sodium cromoglicate (Intal Forte) and nedocromil (Tilade) MDIs have been largely used to prevent asthma
caused by allergens, however, they are rarely used in long-term management.3 Both inhalers are to be discontinued
in New Zealand and there are no other suppliers. Nedocromil inhalers are expected to be out of stock in late July, 2020,
and supplies of sodium cromoglicate inhalers are expected to last until late October, 2020.
No new patients should be initiated on nedocromil or sodium cromoglicate inhalers and patients currently using these
inhalers should be transitioned to an alternative treatment.
Severe asthma: add-on treatments
Patients with poorly controlled asthma at Step 3 require review by a respiratory physician.2 Treatment
options that might be recommended include:2
Increasing ICS beyond standard dosing may be considered for patients with asthma that is poorly controlled
despite lower dose treatment taken optimally.3 This is only appropriate for a small number of patients.3 High
dose ICS treatment, e.g. > 400 micrograms per day of budesonide, > 250 micrograms per day of fluticasone propionate
or > 100 micrograms per day of fluticasone furoate, can be achieved by increasing the number of actuations in the patient’s
maintenance treatment. Patients who are using AIR therapy should not be prescribed the 400/12 Symbicort Turbuhaler.
Oral corticosteroids taken at the lowest effective dose for maintenance treatment, e.g. prednisone ≤ 7.5
mg/day, may be considered for limited periods for patients with severe asthma, although the risk of adverse effects developing
with long-term use is significant.3 Tapering on withdrawal is required if treatment is longer than two weeks.3
Tiotropium, an inhaled long-acting muscarinic antagonist (LAMA), may be an appropriate additional
treatment for people with features of asthma and COPD, i.e. asthma COPD overlap syndrome (ACOS). Although tiotropium
is indicated as an adjunctive treatment for patients with asthma, it is only funded as an endorsed prescription for
those with a diagnosis of COPD. Tiotropium is associated with modest reductions in exacerbations and small improvements
in lung function.3 Other
LAMAs, e.g. glycopyrronium and umeclidinium, may also be beneficial, however, they are not approved or
funded for patients with asthma.
Further information on ACOS is available from “An update on the pharmacological management of stable COPD” Available
from: https://bpac.org.nz/2020/copd.aspx
Three biological medicines are funded with Special Authority approval for patients with asthma,
on application from a respiratory physician or clinical immunologist (both the initial application and renewals); omalizumab, mepolizumab and benralizumab.
These medicines target inflammatory pathways in people with severe asthma. Omalizumab may be beneficial for patients with elevated serum
immunoglobulin E antibodies while mepolizumab and benralizumab may be beneficial for patients with elevated blood eosinophil counts.2 All three medicines are delivered by subcutaneous injection. Omalizumab and mepolizumab are generally administered every four weeks, although some
patients taking omalizumab require two-weekly injections.22 Benralizumab is administered every four weeks for three doses and then every eight weeks for maintenance.22 Patients taking any of these medicines may be at increased
risk of parasitic infections and there is a small risk of hypersensitivity reactions.22
Funding changes for fluticasone and fluticasone with salmeterol inhalers
From 1 September, 2020, the Floair brand of fluticasone metered dose inhaler (MDI) and the RexAir brand of fluticasone
with salmeterol MDI will no longer be funded.23 The Flixotide brand of fluticasone MDI and the Seretide brand
of fluticasone with salmeterol MDI will become the only funded inhalers for these medicines until at least June, 2023.23
This decision does not affect other inhalers containing fluticasone, i.e. fluticasone with salmeterol and fluticasone
furoate with vilanterol.
