Published: 10 July 2020 | Updated: 25 July 2024 | What's changed?
25 July 2024 Funding information updated in Table 1
The recently updated Asthma and Respiratory Foundation NZ guidelines (2020) did not contain any major changes to the
diagnosis or management of asthma in children aged 5–11 years. A SABA reliever used as needed (without an inhaled corticosteroid
[ICS]) continues to be recommended in children who are on Step 1 (Figure 1). This is in contrast to the updated guidelines
for adolescents and adults, which no longer recommend use of SABA alone (to be covered in a future article).2
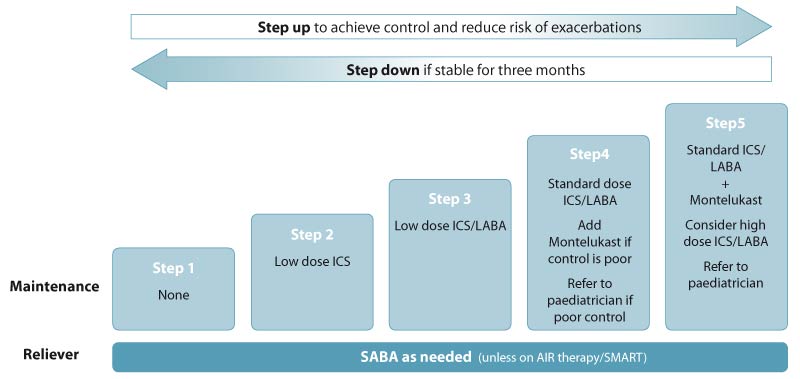
Figure 1. Stepwise treatment for managing asthma in
children aged 5–11 years. Adapted from the Asthma and Respiratory Foundation
NZ guidelines (2020).1 See Table 1 for dose definitions. N.B. “High” dose is double the standard dose.
Stepwise treatment of asthma in children aged 5–11 years1
Pharmacological management of asthma in children follows a stepwise progression where stepping treatment up or down
is guided by symptom control and risk of exacerbations.1 Before stepping up, check inhaler technique (including
use of a spacer), adherence, understanding of the management plan and any barriers to its implementation.1 Once
symptoms have been well-controlled for at least eight weeks, consider stepping down and reassess control after 12 weeks.1
Step 1 is for children with intermittent symptoms and involves as needed use of a SABA, without maintenance treatment.
Step 2 is for children who have symptoms >2 times/week, use a reliever >2 times/week, had regular
night waking with symptoms in the past month or had an exacerbation requiring oral corticosteroids in the past year. This
step involves the addition of a daily low dose ICS (see Table 1 for dose definitions). Montelukast, a leukotriene receptor
antagonist, may be considered as an alternative to ICS, but ICS are generally more effective.
Step 3 is for children with uncontrolled symptoms despite optimal treatment at Step 2. This step involves
the addition of a LABA in combination with an ICS. LABAs (with ICS) should only be initiated when the child is clinically
stable. If the LABA is ineffective or symptoms worsen after starting it, treatment should be stopped.
Step 4 is for children with uncontrolled symptoms despite optimal treatment at Step 3. This step involves
an increase from low dose ICS/LABA to standard dose ICS/LABA (Table 1). Montelukast may be added if control remains poor.
If control at Step 4 is poor, refer for paediatric assessment.
Step 5 is for children with uncontrolled symptoms despite optimal treatment at Step 4. This step involves
standard dose ICS/LABA and montelukast, if the child is not already taking this. High dose ICS/LABA may be considered
(Table 1). The child should be referred for paediatric assessment.
N.B. An anti-inflammatory reliever (AIR) regimen that includes maintenance budesonide/formoterol (also known as Single
combination ICS/LABA inhaler Maintenance And Reliever Therapy [SMART]) may be considered for children who are poorly controlled
on Steps 3–5 on advice from a paediatric respiratory specialist.1 AIR therapy/SMART is unapproved in children.
Table 1. Low and standard ICS doses for children recommended in the Asthma and Respiratory Foundation NZ guidelines.1
ICS
(funded brand name) |
Low dose |
Standard dose |
Beclomethasone dipropionate
(Beclazone) |
200 micrograms/day
Prescribe:
- Beclazone 100, one actuation, twice daily
OR
- Beclazone 50, two actuations, twice daily
|
400–500 micrograms/day
Prescribe:
- Beclazone 100, two actuations, twice daily
OR
- Beclazone 250, one actuation, twice daily
|
Beclomethasone dipropionate extrafine
(Qvar) |
100 micrograms/day
Prescribe:
- Qvar 50, one actuation, twice daily
|
200 micrograms/day
Prescribe:
- Qvar 100, one actuation, twice daily
|
Budesonide
(Pulmicort) |
200 micrograms/day
Prescribe:
- Pulmicort 100, one actuation, twice daily
|
400 micrograms/day
Prescribe:
- Pulmicort 200, one actuation, twice daily
OR
- Pulmicort 100, two actuations, twice daily
|
Fluticasone propionate
(Flixotide*) |
100 micrograms/day
Prescribe:
- Flixotide 50, one actuation, twice daily
|
200–250 micrograms/day
Prescribe:
- Flixotide 50, two actuations, twice daily
OR
- Flixotide 100, one actuation, twice daily
OR
- Flixotide 125, one actuation, twice daily
|
* Prescribe metered dose inhaler (MDI) unless child is able to use the dry powder inhaler (accuhaler)
Oral corticosteroids are indicated for moderate and severe acute asthma
Initial treatment with SABA, delivered via a metered dose inhaler and spacer, and oral corticosteroids is likely to
be sufficient for most children presenting with acute moderate or severe asthma.1 While the evidence of benefit
of oral corticosteroid treatment in children aged under five years is limited, in older children with acute asthma, oral
corticosteroids reduce the need for hospital admission and the risk of relapse.3 If required, give oral prednisolone
(liquid) or prednisone (tablet) at 1–2 mg/kg per day, up to maximum of 40 mg daily, for 3–5 days.1 Tapering
before stopping treatment is not necessary for short courses. Some asthma action plans for school-age children may include
directions for when to initiate a course of oral corticosteroids prescribed for use at home. However, children who require
frequent or continuous use of oral corticosteroids (more than 14 days in 12 months) should be referred for paediatric
assessment.1
The Asthma and Respiratory Foundation NZ Child Asthma Guidelines 2020 are available from:
https://www.nzrespiratoryguidelines.co.nz/childguidelines-654716.html
For information on the pharmacological management of asthma in adolescents and adults, see: https://bpac.org.nz/2020/asthma.aspx
