Published: 18 November 2022 | Updated 21 July 2023
What's changed?
21 July 2023 Updated nomenclature relating to fatty liver disease (MASLD)
Key practice points:
- A liver function test panel generally consists of around seven individual biochemical analyses, including:
- Enzymes associated with liver injury/hepatocyte necrosis (alanine aminotransferase [ALT] and aspartate aminotransferase [AST])
- Enzymes associated with liver or bile duct stimulation (alkaline phosphatase [ALP] and γ-glutamyl-transferase [GGT])
- Proteins associated with liver synthetic function (e.g. albumin and prothrombin time/international normalised ratio [INR])
- Markers of biliary drainage (bilirubin)
- There are varied reasons for requesting LFTs, but this panel should only be used when specifically indicated by the clinical picture. Main indications are patients with risk factors for viral hepatitis (particularly chronic hepatitis B or C; see main text for further information), alcohol-related liver disease (i.e. excessive alcohol intake) or metabolic associated liver disease (e.g. obesity, type 2 diabetes, hypertension, hyperlipidaemia), as well as monitoring the effects of certain medicines (e.g. methotrexate).
- While LFTs are important to diagnose and monitor liver damage, many markers are not direct measures of liver function and abnormal results do not always indicate liver-specific disease. Results must be interpreted in the context of patient-specific characteristics, current clinical situation, medical history and previous LFT results.
- There are two main patterns of LFT abnormalities that can provide clues about the location or type of liver dysfunction:
- 1. Hepatocellular injury – reflected by increased aminotransferase levels (ALT and AST). The AST:ALT ratio may be helpful in determining both the aetiology and the stage of liver disease (see main text).
- 2. Cholestasis – reflected by increased ALP and GGT levels and, if severe, elevated bilirubin level. This pattern reflects conditions that reduce either the transport of bile from the hepatocyte into the canaliculi (intrahepatic cholestasis) or obstruction of flow of bile through the extrahepatic bile ducts into the gut (extrahepatic biliary obstruction).
- Mixed hepatocellular injury/cholestasis patterns are also possible, as severe hepatocellular injury causes liver swelling which blocks the small bile ductules within the liver, preventing flow of bile from the liver (intrahepatic cholestasis). This finding is more common in the context of medicine use.
- Isolated bilirubin increases should be further evaluated by requesting a fractionated/split bilirubin test (i.e. to estimate the proportion that is unconjugated/indirect) because this may be due to causes other than hepatocellular injury or cholestasis
- Both acute and chronic liver disease can lead to the development of complications of liver failure and portal hypertension
- Liver failure is associated with reduced liver synthesis of proteins, such as albumin or coagulation factors (resulting in increased prothrombin time or INR). Synthetic liver failure can be either acute or chronic liver failure. Acute liver failure is usually caused by acute hepatitis (usually HBV infection), or medicine-induced liver damage (especially paracetamol). Chronic liver failure is usually caused by decompensated cirrhosis (any aetiology). This can occur in the context of either hepatocellular injury or cholestatic LFT patterns and these patients require referral for urgent ultrasound or acute secondary care review.
- Portal hypertension is associated with low platelet counts, ultrasound findings of enlarged spleen, portal vein and ascites or varices
- Asymptomatic patients with borderline LFT abnormalities often do not require immediate follow-up testing, particularly if there is a likely modifiable cause present, e.g. alcohol misuse, medicine changes or dose increases, acute viral illness; consider repeating LFTs within three months
- If the cause of LFT derangement is uncertain or if established liver disease is suspected, a tiered approach to subsequent laboratory testing and other investigations is generally recommended, informed by the LFT pattern and patient characteristics (see main text for specific recommendations)
- Ultrasound imaging is a first-line investigation if cholestasis is suspected based on LFT results. If hepatocellular injury is suspected and the patient does not have concerning symptoms/features, ultrasound is a second-tier investigation after investigating the most likely causes (listed above).
When healthy, the liver mediates a wide range of essential bodily processes, including carbohydrate, protein (amino acid) and lipid metabolism and storage, waste product and ingested toxin breakdown/excretion, red blood cell (RBC) storage, and hormone and lymphatic fluid production.1 All other organs in the body are dependent on the liver for optimal condition and performance, however, this high demand and functional interconnectedness also places it at increased risk of disease.1 This includes potentially transient disturbances in function caused by acute injury (e.g. medicine exposure, acute viral illness) through to established disease associated with progressive pathological changes (i.e. the spectrum of disease leading from steatosis to cirrhosis; Figure 1).
Except in instances of acute failure, liver disease usually progresses silently over years or decades, with no obvious signs or symptoms occurring until complications associated with chronic failure develop.2, 3 In 2019, there were 158 deaths caused by liver cirrhosis in New Zealand (3.3 deaths per 100,000 people) and 188 deaths due to liver cancer (6.0 deaths per 100,000 people).4 Māori, Pacific and Asian peoples are considerably more likely to develop chronic liver disease compared with those of European/Other ethnicity.5 This is likely to be related to a higher prevalence of associated risk factors, e.g. metabolic syndrome, chronic viral hepatitis.
Given that the liver is the only internal organ capable of substantial natural tissue regeneration to levels required for stable body system functioning,6 early identification of liver injury is an important clinical objective so that corrective lifestyle changes or targeted interventions can be applied.
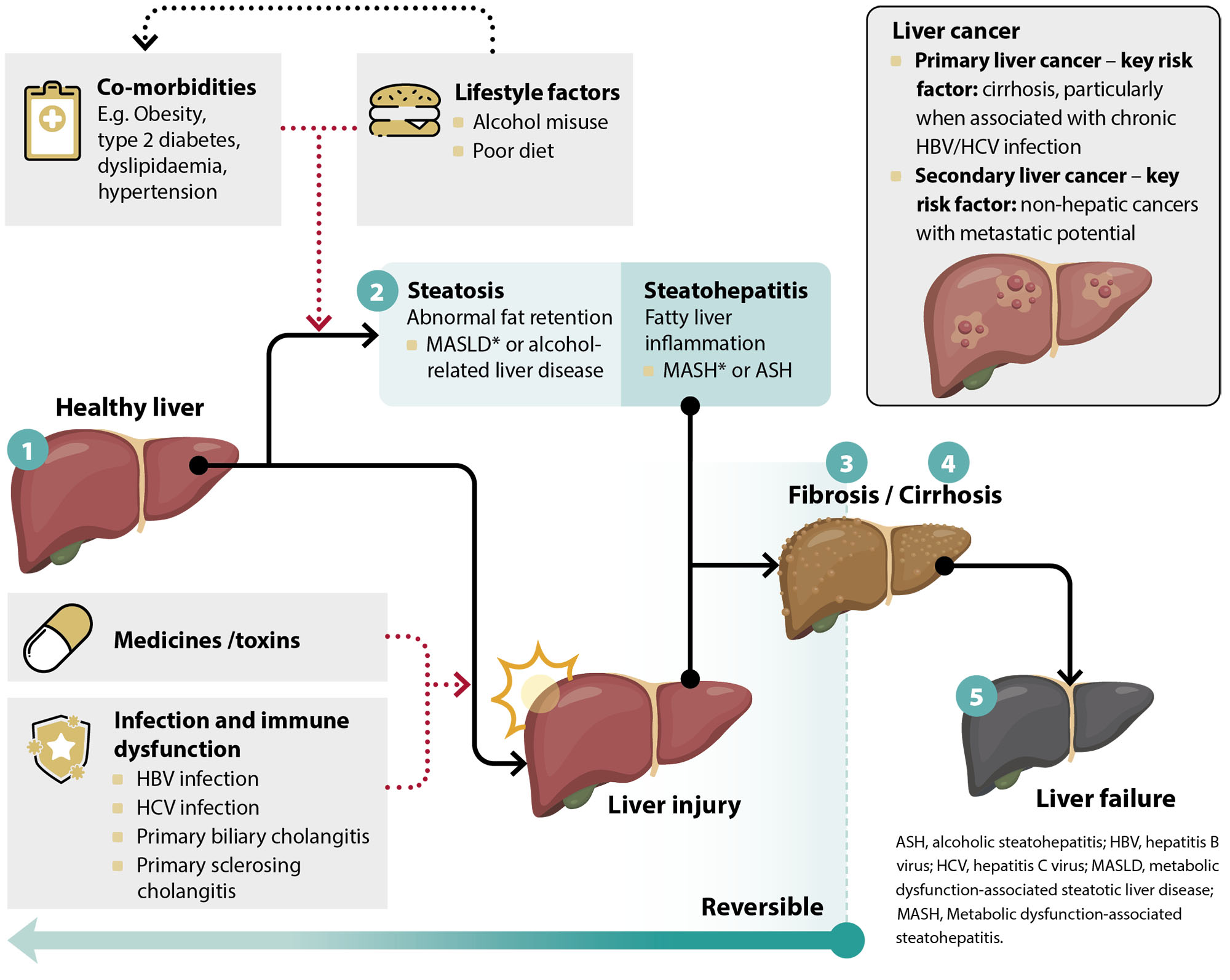
Figure 1. Physiological spectrum of progressive liver disease. Adapted from Tripathi et al, 2018.3
* Nomenclature update (2023): The American Association for the Study of Liver Diseases (AASLD) has announced a new overarching term to describe the various forms of steatosis influenced by metabolic processes. The previously used term non-alcoholic fatty liver disease (NAFLD) – which is also referred to in the literature as metabolic dysfunction-associated fatty liver disease (MAFLD) – will now be labelled metabolic dysfunction-associated steatotic liver disease (MASLD; pronounced 'ma-zuld').
MASLD is used to describe patients who have hepatic steatosis (i.e. fat accumulation in the liver), in addition to at least one of five cardiometabolic risk factors, without other potential causes. The thresholds for these cardiometabolic factors differ between adults and children. Analysis of European registry records indicates that 98% of patients currently identified as having NAFLD/MAFLD fit the new criteria for MASLD.
If an additional cause of hepatic steatosis is identified outside of a confirmed cardiometabolic driver, the patient is labelled as having a combination aetiology, e.g. MetALD in the case of alcohol, which is distinct from alcohol-related liver disease. If no cardiometabolic criteria is fulfilled, then the patient is defined as having either:
- Liver disease defined by any other cause identified, e.g. alcohol-related liver disease, drug-induced liver disease
- Cryptogenic steatotic liver disease if no other cause is identified. Alternatively, if there is strong clinical suspicion of metabolic dysfunction despite the absence of cardiometabolic risk factors, the patient can be labelled as having “possible MASLD” while awaiting the results of additional testing.
Metabolic dysfunction-associated steatohepatitis (MASH) is also now accepted as the replacement term for non-alcoholic related steatohepatitis (NASH).
For further information, see: Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023; Published online ahead of print. doi:10.1097/HEP.0000000000000520.
Liver function tests (LFTs) involve a panel of laboratory analyses performed on a single blood sample to assess the organ’s performance. Table 1 outlines the most common markers used in LFTs in New Zealand and their biological significance. However, the combination of tests within a LFT panel may differ between laboratories and regions.
While the term “liver function test” is widely used, it is potentially misleading in that many of the tests are not a direct measure of liver function and may be abnormal in patients with an otherwise “healthy” or functional liver. This presents a challenge for primary care clinicians, particularly given that elevated liver enzyme levels are estimated to occur in up to 9% of asymptomatic people.7
The clinical picture is further complicated by several other factors:8–10
- Some abnormalities are transient and self-resolve within several weeks, whereas others reflect a persistent underlying issue
- Various non-hepatic diseases can influence LFT marker levels
- Liver-specific causes of disease do not always correlate with a consistent LFT marker profile and some patients have LFT abnormalities earlier in the physiological spectrum of disease than others (Figure 1)
- There is a natural age-associated decline in liver functioning over time
Therefore, after identifying any LFT “abnormality” (see: “Interpretation of LFT results should be individualised”), its clinical significance must then be assessed in the context of the patient’s characteristics, their current clinical situation, medical history and previous test results. This information can then provide clues to refine subsequent investigations. As such, learning to recognise certain biochemical signatures in the context of specific patient types becomes an important skill that general practitioners develop over time.
Table 1. Common markers used in liver function tests (LFTs).* 11
|
LFT marker (results of tests with matching colours often correlate with each other) |
Site of production |
Usual function |
Notes |
| Common default laboratory LFT markers |
Alanine aminotransferase (ALT)†● |
Predominantly liver |
Intracellular enzymes involved in glucose production and amino acid metabolism, allowing them to be used for cellular energy production |
Highly specific for liver injury |
| Aspartate aminotransferase (AST)†● |
Liver, heart, skeletal muscle, kidney, brain, RBCs |
Less sensitive and less specific compared with ALT as it is also produced in other organs and tissues |
| Alkaline phosphatase (ALP)● |
Predominantly liver and bone, also in bile ducts, kidney, intestine, placenta |
A group of enzymes that co-ordinate various bodily functions; in the liver ALP is associated with protein breakdown |
More specific than GGT for liver disease; ALP is induced by bile acids, and ALP elevation indicates biliary obstruction. Levels are higher in childhood due to bone turnover. |
| γ-Glutamyl-transferase (GGT)● |
Liver and bile ducts, also present in cell membranes of multiple other tissues, e.g. pancreas, kidneys |
Enzyme present in cell membranes; catalyses transfer of amino acids across the membrane and is involved in the metabolism of various molecules |
Considered more sensitive than ALP; however mild elevations are non-specific, and isolated increases are rarely indicative of liver disease |
| Bilirubin |
Bone marrow, liver |
Initial testing usually reports total bilirubin, which includes both unconjugated/indirect and conjugated/direct fractions. The unconjugated form is increased by RBC breakdown (e.g. haemolysis), if hepatic uptake is reduced (e.g. medicine-induced or when hepatic blood flow decreases [such as in heart failure]), or if hepatic conjugation is impaired due to hereditary deficiency in the UGT enzyme (i.e. Gilbert’s syndrome, which is found in approximately 5% of people). In contrast, conjugated hyperbilirubinaemia is usually caused by impaired liver processing/bile flow (e.g. hepatitis, medicine-induced cholestasis, or biliary obstruction by gall stones or malignancy). For further information, see: “Isolated hyperbilirubinaemia”. |
| Serum albumin |
Exclusively produced by the liver |
A protein involved in maintaining blood oncotic pressure (the pressure exerted by plasma proteins on capillary walls); also functions as a ligand transporter |
Usually constitutes 50% of circulating proteins. Levels may still be normal in patients with severe acute liver damage as the half-life of albumin in plasma is roughly 20 days. |
| Total protein |
Includes albumin immunoglobulins and other carrier proteins present in the blood; a variable proportion is synthesised in the liver |
Changes in the total protein are non-specific as there may be increases and decreases in different components, e.g. chronic severe inflammatory liver disease may cause low albumin and other liver globulins but raised levels of immunoglobulins |
| Additional |
Prothrombin time/International normalised ratio (INR) |
A reflection of liver synthesis of vitamin K dependent clotting factors II (prothrombin), VII, IX, X. A prothrombin time test measures the time taken for clotting to occur in a sample, while the INR is a calculation based on the prothrombin time results to ensure standardisation between laboratories. |
Outside the context of warfarin monitoring, a raised INR may indicate decreased liver synthetic capacity or cholestasis with reduced vitamin K absorption. Levels rise quickly with acute liver disturbance because of the short half-life of the clotting factors. |
*Community laboratories will provide reference ranges for LFT markers. For an example of
LFT reference intervals stratified by sex and age, see: www.labtests.co.nz/wp-content/uploads/sites/2/2019/11/Liver_Function.pdf.
† Sulfasalazine and sulfapyridine use may lead to false negative aminotransferase results. Some laboratories do not include AST in the initial LFT panel as ALT is more specific.
Opportunistic liver function testing is not indicated for asymptomatic people without risk factors.2, 12 As noted, healthy people can have mildly abnormal LFT results, and unwarranted testing in response to isolated non-specific symptoms (such as brief periods of fatigue) may prompt unnecessary patient concern or investigation/treatment escalation if false positive results occur.13
In general, the main indications for liver function testing include:2, 12
 Risk factors for viral hepatitis (see: “A spotlight on the key causes of liver disease” for a more comprehensive list):
Risk factors for viral hepatitis (see: “A spotlight on the key causes of liver disease” for a more comprehensive list):
- Hepatitis C, e.g. injecting drug users, children of mother with hepatitis C, immigrants from high-risk areas, recipients of blood transfusion prior to 1992
- Hepatitis B, e.g. Māori, Pacific or Asian people born prior to 1990 (when universal neonatal vaccination was introduced), high-risk sexual practices especially in men who have sex with men (MSM)
- Hepatitis A, e.g. travel to countries with poor sanitation or a lack of safe water within last two months, recent local outbreak, MSM
- Other rarer causes of viral hepatitis include Epstein Barr virus and travel-related viruses
- Risk factors for metabolic
dysfunction-associated steatotic liver disease (MASLD; previously known as non-alcoholic fatty liver disease [NAFLD] or metabolic dysfunction-associated fatty liver disease [MAFLD]), e.g. obesity, type 2 diabetes, dyslipidaemia, hypertension
- Risk factors for alcoholic liver disease, e.g. excessive alcohol intake
- Risk factors for immune-mediated liver diseases (e.g. primary biliary cholangitis, primary sclerosing cholangitis, autoimmune hepatitis), including other pre-existing autoimmune diseases or inflammatory bowel disease
- Monitoring the effects of medicines that can affect liver function or that are hepatotoxic, e.g. methotrexate, sodium valproate
- To exclude liver disease when a patient has a pattern of persistent non-specific symptoms with no obvious identifiable cause or link or if they have features such as jaundice or ascites on examination
- Family history of liver disease or co-morbidities likely to influence liver function (see above)
For further information regarding the potential causes underpinning these indications, see: “Clues that could reveal a potential cause for abnormal LFTs”
Reference ranges for LFTs will be provided by the community laboratory performing the analysis. An “abnormal” LFT result is a value that falls outside the range expected for 95% of a healthy reference population.8 This reference range can differ based on factors such as the patient’s sex and age.
 For an example of LFT reference intervals stratified by sex and age, see: www.labtests.co.nz
For an example of LFT reference intervals stratified by sex and age, see: www.labtests.co.nz
As a general guide, marker abnormalities are usually considered:7, 8
- “Borderline” if they are < 2 times the upper limit of normal (ULN)
- “Mild” if they are 2 – 5 times the ULN
The line that then separates “moderate” and “marked” LFT increases differs between international guidelines, with some labelling moderate as results between 5 – 10 times the ULN,7, 8 whereas others consider the threshold to be > 15 times the ULN.8, 14 In reality, this distinction is arbitrary, and the magnitude of derangement in isolation is not a reliable predictor of prognosis.2 Instead, the clinical significance of any change(s) should be interpreted in the context of (Figure 2):
- The specific patient’s characteristics, current clinical situation, medical history and risk factors for liver disease
- The overall pattern of LFT abnormality and any past results
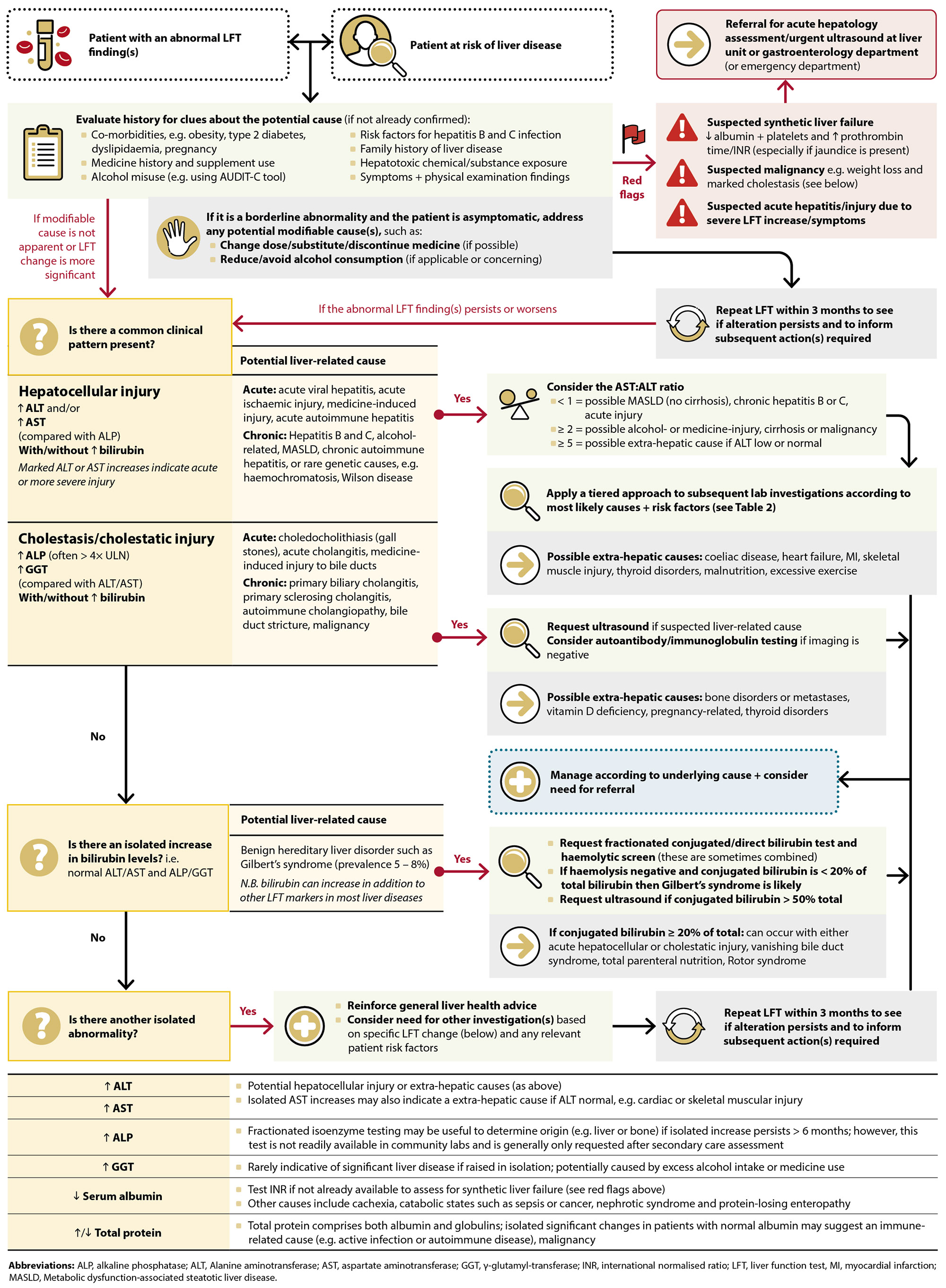
Figure 2. A pathway for interpreting abnormal LFT results in primary care.2, 7–9
N.B. This algorithm is mostly applicable to stable patients with either no symptoms or mild symptoms. The presence of significant clinical features should increase suspicion of acute or advanced liver disease or extra-hepatic conditions.
Common causes of abnormal LFTs
Co-morbidities. Obesity, type 2 diabetes, dyslipidaemia and hypertension share similar lifestyle risk factors with liver disease and promote its progression (see: “A spotlight on the key causes of liver disease”).2 In some cases the relationship is bidirectional, with liver disease also impacting on co-morbidity outcomes, e.g. type 2 diabetes.15 Other potential co-morbidities associated with liver disease include autoimmune conditions (e.g. coeliac disease), inflammatory bowel disease or a history of cancer, i.e. metastases being deposited in hepatic tissue.
Alcohol consumption. Consider past and present alcohol use (see: “A spotlight on the key causes of liver disease”). While even short periods of alcohol misuse can cause liver damage, the risk is highest in people who drink heavily over several years.16
Viral hepatitis. Review for risk factors of viral hepatitis (see: “Indications for requesting LFTs” in this article, or: “A spotlight on the key causes of liver disease” for more comprehensive lists of risk factors).
Medicine history and supplement use. Numerous medicines are extensively metabolised within the liver, and some are directly hepatotoxic.1 In any patients with an abnormal LFT result(s), consider any medicines that have been recently initiated or dosage changes. Also ask about use of any over-the-counter (OTC) medicines or herbal or dietary supplements.1, 17 See: “Always consider a possible pharmacological cause”.
Rare causes of abnormal LFTs
Family history of liver disease. Certain genetic conditions are associated with liver damage, e.g. haemochromatosis, alpha-1 antitrypsin deficiency, Wilson’s disease (rare).2, 7
Exposure to hepatotoxic chemicals. If relevant, ask about potential occupational or recreational exposure to hepatotoxic chemicals or industrial products, particularly in workers such as painters, builders and factory workers. Some common industrial solvents and epoxy resin hardeners are associated with hepatocyte injury/cholestasis, e.g. dimethylformamide, dimethylacetamide, trichloroethylene.18
How can symptoms and signs add to the clinical picture?
Acute liver disease symptoms. Acute hepatitis from any cause is usually associated with non-specific symptoms of anorexia, fatigue and nausea. In severe cases, acute hepatitis can cause right upper quadrant abdominal discomfort, vomiting and jaundice.2, 19 Acute cholangitis from any cause is usually associated with the triad of (1) right upper quadrant abdominal pain, (2) fever and (3) jaundice.
Practice point: Cholecystitis is usually identified according to clinical presentation rather than LFT results and patients require acute referral and ultrasound if it is suspected. The most common laboratory findings in patients with uncomplicated cholecystitis are leukocytosis and increased serum amylase levels. LFTs are often normal or associated with increased ALP levels. For further information, see: bpac.org.nz/bpj/2014/june/gallstones.aspx
Chronic liver disease symptoms. In the early stages, most people with chronic liver disease are either asymptomatic or have mild non-specific symptoms such as fatigue.2 However, in the advanced stages, symptoms can include weight loss (from malnutrition), weight gain with swollen legs (from fluid retention), abdominal swelling (from ascites), gastrointestinal bleeding (from varices), sleep disturbance, drowsiness or confusion (from encephalopathy) or jaundice.19, 20
Physical examination. Physical examination findings are often normal in patients with LFT derangement; the presence of abnormal findings should increase suspicion of more advanced or severe liver disease.8 For example:8, 14
- Hepatomegaly is more common in alcohol-related liver disease, haemochromatosis and primary biliary cirrhosis than other liver diseases. Hepatomegaly may also be caused by liver congestion (e.g. due to heart failure or hepatic venous obstruction) or malignant infiltration (particularly lymphoma and hepatocellular carcinoma).
- Jaundice may indicate biliary obstruction, acute hepatitis or advanced liver disease
- Skin hyperpigmentation and arthralgias may be indicative of haemochromatosis
- Muscle wasting, spider telangiectases (also known as spider naevus or spider angiomas), palmar erythema, gynaecomastia, testicular atrophy, ascites and splenomegaly may indicate cirrhosis
- Asterixis (hepatic flap/flapping tremor) is a distinctive sign of hepatic encephalopathy
- Kayser-Fleischer rings and neurologic motor abnormalities are rare features of Wilson’s disease
Always consider a possible pharmacological cause
While it is uncertain what proportion of abnormal LFT results are medicine-related in asymptomatic patients, at least 20% of all acute liver failure events are associated with medicine use.21 When reviewing the history of patients with abnormal LFT findings, any newly initiated medicines should be considered a potential cause until proven otherwise (also see alternative medicines and other substances below).
- The patten of LFT derangement linked to medicine use can vary; sometimes being reflective of hepatocellular injury, cholestatic injury or a combination of both (see below and also “Consider whether a common LFT pattern is present”)22
- The time to liver toxicity can differ depending on the medicine and patient; medicines with dose-dependent effects can cause toxicity within short periods of time (e.g. hours to days of exposure), whereas those with idiosyncratic effects (dose-independent) may not cause toxicity until weeks or months after exposure.22
Frequently prescribed medicines in primary care that influence liver function:1, 20, 22
- Paracetamol. Hepatotoxicity is dose-dependent; scenarios include incorrect dosing, prolonged use, inadvertent use of two different medicines containing paracetamol, intentional overdose. Paracetamol-related liver injury typically involves marked aminotransferase elevations (ALT and AST), which may be extreme in cases of acute overdose (e.g. > 10,000 U/L).20
- Antibiotics, e.g. amoxicillin + clavulanic acid, flucloxacillin, erythromycin. Liver damage is often dose-independent and transient LFT changes can occur even with standard dosing.20 Sometimes a course of antibiotics can cause abnormal LFTs several weeks/months after completion (in addition to features such as persistent jaundice).
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Anticonvulsant medicines, e.g. phenytoin, carbamazepine, sodium valproate
- Immunomodulatory medicines, e.g. methotrexate, azathioprine
- Other medicines, including allopurinol, amiodarone, isotretinoin, isoniazid
To assess whether a medicine undergoes significant hepatic metabolism or can be used in patients with hepatic impairment, refer to the New Zealand Formulary
The investigation should not just be limited to prescribed medicines. Also consider:
- OTC medicine use, particularly paracetamol or paracetamol-containing products (such as “cold and flu” preparations) and NSAIDs
- Dietary supplements, e.g. turmeric, green tea extract
- Complementary and alternative medicines, including traditional Chinese medicines (e.g. Ba Jiao Lian [Dysosma pleianthum], Chi R Yun [Breynia officinalis], Jin Bu Huan [Lycopodium serratum], Ma Huang [Ephedra sinica], and Shou Wu Pian [Polygonum multiflorum]), Indian (ayurvedic) treatments, Polynesian traditional/herbal remedies and Rongoā Māori/Rākau preparations (e.g. those involving Usnea lichen)24
- Recreational drug use (especially ecstasy/MDMA and methamphetamine)
If any potential pharmacological cause* is identified, the subsequent action will depend on the medicine, balancing (1) its importance to the patient’s treatment and (2) the magnitude of the LFT derangement or perceived clinical risk. Consider whether a medicine/supplement can be withdrawn or a substitute can be used, particularly if the abnormality is severe and it is clinically reasonable to do so.25 Alternatively, a dose reduction may be warranted for essential medicines.25 Assuming the patient is stable and does not require secondary care review, repeat LFT testing should be undertaken within three months to assess whether the abnormality has corrected.25
*Some medicines have a specific detoxification protocol, e.g. paracetamol overdose requires administration of intravenous acetylcysteine in patients at risk of hepatotoxicity (for further information, see: www.mja.com.au/journal/2020/212/4/updated-guidelines-management-paracetamol-poisoning-australia-and-new-zealand).
Read more about possible LFT patterns associated with specific medicines and supplements20, 23
Hepatocellular injury
(↑ ALT and/or ↑ AST ± ↑ bilirubin) |
Cholestatic injury
(↑ ALP and ↑ GGT ± ↑ bilirubin) |
Mixed pattern |
Paracetamol
Allopurinol
Sodium valproate
Disulfiram
Isoniazid
Green tea extract |
Oral contraceptives
Amoxicillin + clavulanic acid
Cephalosporins
Erythromycin
Flucloxacillin
Rifampicin
Tricyclic antidepressants
Anabolic steroids |
Carbamazepine
Phenytoin
Lamotrigine
NSAIDs |
N.B. These are examples of medicines which cause more predictable LFT changes; other medicines can induce liver damage, but the associated LFT derangement is variable.

Individual LFT marker changes are not usually specific to a particular condition; instead, the pattern of LFT marker alteration is usually more informative if a review of the patient’s history does not immediately reveal a likely underlying cause.2 In many cases, this pattern will be one of the first pieces of evidence to capture a clinician’s attention, which will then guide subsequent investigations. While any liver disease can paint a complex biochemical picture, there are usually two key patterns of LFT abnormality which reflect distinct types and locations of liver injury (Figure 2): hepatocellular injury and cholestasis.
Pattern 1: Hepatocellular injury (increased aminotransferases ± increased bilirubin)
When liver cells are damaged by processes such as necrosis or inflammation, their membranes become more permeable, which can lead to the release of intracellular enzymes into the bloodstream.2 The most notable markers released through this process are the aminotransferases (Table 1): alanine aminotransferase (ALT) and aspartate aminotransferase (AST).2 Disproportionate serum elevations for either enzyme compared with ALP are the most common LFT abnormalities in this context; ALT and AST level changes frequently correlate with each other.2 However, ALT is considered to be more specific for liver dysfunction as this is the predominant site of its production.2 AST is also abundant in other organs and tissues (including skeletal, cardiac and smooth muscle), so isolated increases may indicate extra-hepatic conditions, e.g. myocardial infarction or myositis.2
A wide range of processes can damage liver cells, which may become apparent through a review of the patient history and risk factors, or after consideration of the AST:ALT ratio (see: “Consider the AST:ALT ratio”). Key causes of hepatocellular injury include metabolic dysfunction-associated steatotic liver disease (MASLD), medicine or alcohol-induced liver damage and viral hepatitis.7 Rarer causes include haemochromatosis, autoimmune diseases and endocrine disorders.7
For further information, see: “A spotlight on the key causes of chronic liver disease”
If hepatocellular injury is suspected, a tiered approach to subsequent testing is recommended, informed by patient-specific characteristics (Figure 2 and Table 2).2, 7–9 For example, if a patient has increased aminotransferases and risk factors for viral hepatitis, testing for hepatitis B surface antigen (HBsAg) and anti-hepatitis C virus antibodies (anti-HCVAb) would be key initial investigations as hepatitis B and C are the most common viral triggers. If these tests are negative, investigating other potential viral causes could be considered (Table 2).2
Table 2. Recommendations for possible subsequent testing and interpretation of results in
stable patients with elevated aminotransferase levels (ALT or AST).2, 7–9
| Test |
Abnormality |
Interpretation or potential cause |
| First tier |
|
|
| Full blood count (FBC) |
Macrocytosis* |
Possibly due to excessive alcohol intake, particularly if GGT also raised |
| Thrombocytopenia* |
Portal hypertension (possible hypersplenism) which may also be associated with ultrasound findings of enlarged spleen, portal vein and ascites or varices. Also common in chronic liver disease. |
| HbA1c or fasting glucose |
Elevated (e.g. HbA1c > 41 mmol/mol
or fasting glucose > 5.5 mmol/L) |
Indicates glucose intolerance conditions, e.g. type 2 diabetes and pre-diabetes; MASLD is common in these patient groups |
| Lipid profile |
Abnormal |
Contributor to fatty liver diseases, i.e. alcohol-related liver disease or MASLD |
Iron studies,*
inc. ferritin and TSAT |
Elevated |
Possible haemochromatosis. Check hereditary haemochromatosis (HFE) genotype if repeat testing remains high in fasting† and otherwise well patients |
Hepatitis screening
(HBsAg and anti-HCVAb) |
Positive |
Suggests hepatitis B or C infection. HCV requires confirmation of infection by either detection of HCV RNA or HCV Antigen |
| AUDIT-C screening |
Positive
(score ≥ 3 in female or ≥ 4 in male) |
Suggests potential alcohol misuse; full AUDIT tool assessment should then be undertaken |
| Second tier |
|
|
| Abdominal/ liver ultrasound** |
Echogenic liver, mass or dilated ducts |
Can be used to detect fatty liver, malignancy or gall stones/obstruction |
| Testing for other causes of viral hepatitis, e.g. Hepatitis A, Epstein Barr virus, Cytomegalovirus |
Positive |
Suggests infection with corresponding virus; hepatitis A testing may be a first-tier investigation if the patient reports travel to a country where infection is prevalent or contact with local outbreak or MSM |
| Autoantibodies and Immunoglobulins |
Antimitochondrial antibody (AMA) positive, increased IgM in combination with cholestatic LFTs |
Diagnostic of primary biliary cirrhosis |
| Anti-smooth muscle antibody (SMA)/ anti-liver kidney microsomal (LKM)/anti-liver soluble antigen (SLA)/anti-nuclear antibody (ANA) positive, particularly with elevated IgG |
Probable autoimmune hepatitis |
| Coeliac serology screen |
Positive |
Suggestive of coeliac disease-related liver damage |
Other tests dictated by clinical context or family history of liver disease, such as:
- Checking alpha-1 antitrypsin levels in people with a family history of deficiency
- Serum/urine copper and caeruloplasmin testing in patients with a family history of Wilson’s disease
|
* If these tests have been performed for other clinical reasons and an abnormality is found, then subsequent LFT testing is usually warranted
† A fasting sample may improve the accuracy of results if there is uncertainty about an abnormal result
** In patients where ultrasound does not identify any underlying cause and the diagnosis remains uncertain, consider referring for further assessment with FibroScan, if needed (see: “FibroScan is an emerging alternative tool to liver biopsy”). Liver ultrasound is first tier investigation if cholestasis is suspected.
 Consider the AST:ALT ratio in patients with elevated aminotransferase levels; this can provide information on the likely cause and stage of chronic liver disease and inform the type of subsequent investigations required.
Consider the AST:ALT ratio in patients with elevated aminotransferase levels; this can provide information on the likely cause and stage of chronic liver disease and inform the type of subsequent investigations required.
In general, an AST:ALT ratio of:8
- < 1 (i.e. AST is less than ALT) is generally indicative of chronic viral hepatitis B or C, MASLD without cirrhosis (see: “A spotlight on the key causes of chronic liver disease”) or an acute hepatocellular injury
- ≥ 2 (i.e. AST is at least two times greater than ALT) is generally found in patients with alcohol-related liver disease, drug-induced liver injury, cirrhosis of any cause, and primary liver cancer
- ≥ 5 should prompt suspicion of a potential extrahepatic cause, particularly if ALT levels are minimally elevated or normal
Pattern 2: Cholestasis/cholestatic injury (increased ALP and GGT ± increased bilirubin)
Cholestasis refers to any condition that causes reduced bile production or flow through the bile ducts from the liver into the duodenum.26 Patients with cholestasis are often clinically asymptomatic, however, those with more severe disease may present with features such as intense pruritus, abdominal pain, jaundice and fatigue.26 For further information, see: “The pathophysiology of cholestasis”.
The hallmark LFT findings for cholestasis include elevated ALP and GGT results compared with aminotransferase levels (Figure 2).2, 26 While most other LFT markers are usually within a normal range, bilirubin levels can also be increased.26 If cholestasis is suspected based on LFT results, a liver ultrasound is recommended; this may help identify features such as obstruction, biliary duct dilation, space-occupying lesions (e.g. a cyst) or malignancy, which will then guide subsequent management decisions.8 If there are no significant ultrasound findings, additional serologic blood tests may be helpful, e.g. antimitochondrial antibody positivity can indicate primary biliary cholangitis as a potential cause.8
Fractionated ALP isoenzyme testing. GGT elevation in parallel with increased ALP levels is the strongest LFT-based predictor of cholestasis.26 However, cholestasis may also be associated with isolated ALP increases (i.e. GGT is normal or only borderline elevated) which can be from either a hepatic or extra-hepatic source (e.g. bone, intestine, placenta during pregnancy). A fractionated ALP isoenzyme test can help in this situation to distinguish whether cholestasis is likely or whether other diagnoses should be considered, e.g. bony metastases, Paget’s disease, Vitamin D deficiency.2, 26 However, this test is not readily available in community laboratories in New Zealand and is generally only requested after secondary care referral. ALP isoenzyme testing is most likely to be helpful for patients with a sustained increase in ALP (> 6 months), and no other cause for the increase is clinically apparent.
The pathophysiology of cholestasis
Accurately diagnosing the underlying cause of cholestasis is important to tailor subsequent management decisions; if left unresolved or ineffectively treated, chronic or severe cases are associated with bile duct loss which inevitably leads to fibrosis and cirrhosis.26
Cholestasis occurs when biliary constituents accumulate beyond the normal limits of clearance. This process in turn contributes to direct cellular damage and accumulation of noxious chemicals, including bile acids. Cholestasis can be sub-classified as being intra-hepatic (e.g. due to hepatocyte injury, intra-hepatic bile duct or bile canaliculi abnormalities) or extra-hepatic (e.g. abnormalities associated with the extra-hepatic ducts, the common bile duct or common hepatic duct):26
- Common causes of intrahepatic cholestasis include acute hepatitis, drug-induced cholestasis (e.g. amoxicillin + clavulanic acid, flucloxacillin). Rare causes include hereditary abnormalities in bilirubin transporters (Dubin-Johnson syndrome, Rotor syndrome, and progressive familial intrahepatic cholestasis). Severe sepsis may also cause canalicular dysfunction which results in intrahepatic cholestasis.
- Common causes of extrahepatic biliary obstruction include gallstones and malignancy – either intraductal obstruction from primary liver cancers (hepatocellular carcinoma or cholangiocarcinoma), or extrinsic compression from pancreatic cancer, lymphoma, or lymph node metastases from breast, colorectal cancers
In cases not caused by obstruction, the heritable risk of cholestasis is most notable during childhood, while an autoimmune trigger (e.g. primary biliary cholangitis and primary sclerosing cholangitis) is more likely in adulthood.26
Synthetic liver failure (decreased albumin and prolonged INR)
While the hepatocellular/cholestatic LFT patterns provide clues regarding the location of dysfunction, overall liver performance can also be estimated by its ability to produce albumin – a protein exclusively synthesised in the liver.2 Particular attention should be given to persistent decreases in albumin over time when considering past test results. However, assessment of this marker is complicated as serum albumin concentrations are affected in other clinical circumstances, e.g. diabetic nephropathy, malabsorption, sepsis, systemic inflammatory conditions.2 In addition, albumin has a long half-life, meaning levels may not be substantially reduced in instances of acute liver injury.11
Requesting an assessment of the patient’s INR (in those not taking warfarin) can be useful to more completely define synthetic function if albumin levels have decreased.2 This provides information on whether pathways of coagulation are impaired; fibrinogen and most of the clotting factors used in this investigation are produced in the liver, so an increased INR further reflects hepatic deficiencies.7, 9 Other features that should raise clinical suspicion of synthetic liver failure include low platelet levels and overt clinical signs of liver disease, such as jaundice.2 Vitamin K malabsorption (usually due to cholestasis) can also increase INR, however, this usually resolves within 24 hours of parenteral vitamin K injection (not oral).
- Practice point: Given that synthetic liver failure reflects a loss of functioning liver mass, referral for urgent ultrasound or acute secondary care review is generally recommended if there is clinical suspicion.
Isolated hyperbilirubinaemia
Serum bilirubin levels are not useful for distinguishing between hepatocellular injury or cholestasis as increases can occur with most types of liver damage.7 However, isolated bilirubin increases in patients with otherwise normal LFT results may warrant further investigation as they can indicate several different conditions of varying clinical significance, ranging from benign inherited liver-related conditions (e.g. Gilbert’s syndrome), to haemolytic disorders or serious defects in liver processing/excretion.
Red blood cell (haemoglobin) breakdown generates unconjugated bilirubin, which is transported to the liver, processed into the conjugated form and excreted into bile (Figure 3).2 However, standard LFT panels generally only report total bilirubin, i.e. both conjugated (direct) and unconjugated (indirect) forms. Therefore, a key follow-up investigation in patients with isolated hyperbilirubinaemia is to request fractionated/split bilirubin testing to measure the conjugated/direct component (allowing for estimation of the unconjugated/indirect form). This approach can help to localise the potential source of marker abnormality:7, 8
- Unconjugated hyperbilirubinaemia (conjugated form is < 20% of total bilirubin) is more likely if production increases (e.g. haemolysis) or liver uptake/conjugation is impaired due to mutation in key processing enzymes (e.g. Gilbert’s syndrome)
- Conjugated hyperbilirubinaemia (conjugated form is ≥ 20% of total bilirubin) can occur with either hepatocellular injury or cholestasis. Other conditions associated with conjugated hyperbilirubinaemia include vanishing bile duct syndrome, the effects of total parenteral nutrition, and rare disorders such as Rotor syndrome and Dubin-Johnson Syndrome. Consider ultrasound if the conjugated fraction is > 50% of the total.
If unconjugated hyperbilirubinaemia is identified, haemolysis/haemolytic anaemia should be ruled out by requesting a haemolytic screen, assessing serum haemoglobin, haptoglobin levels, lactate dehydrogenase and reticulocyte count.2, 7 N.B. Some laboratories may include the fractionated bilirubin test in the haemolytic screen. If these haemolytic tests are negative, Gilbert’s syndrome is most likely the cause, which occurs in 5 – 8% of the population.2 This is generally considered a benign condition, with at least one-third of all people being entirely asymptomatic.2
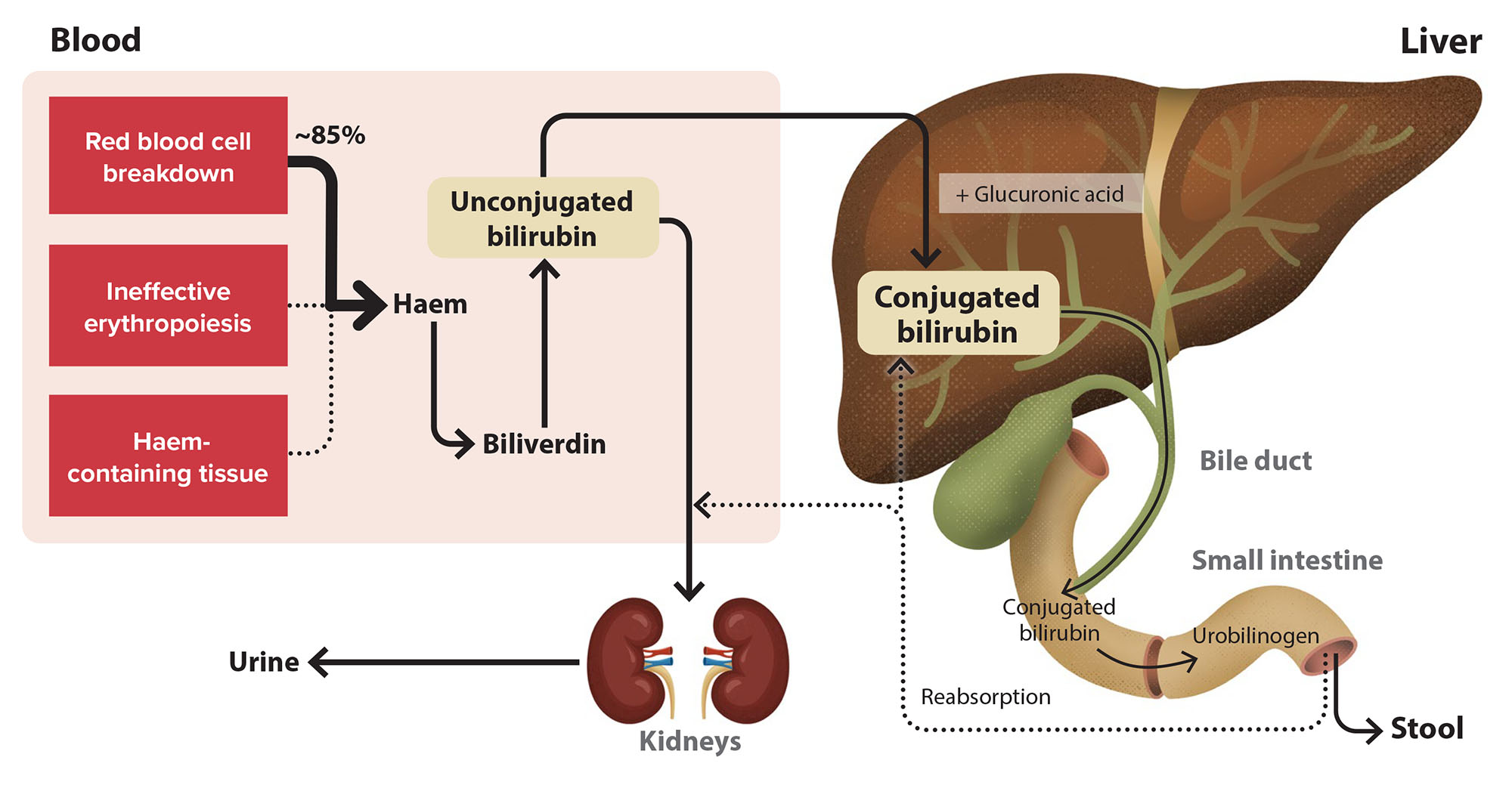
Figure 3. An overview of bilirubin metabolism.2
People with true liver dysfunction are most likely to have multiple abnormal LFT results rather than isolated marker derangement.2 All LFT marker levels can be affected by non-hepatic disease, modifiable risk factors or transiently fluctuate, and therefore an isolated abnormality should increase clinical suspicion of these various possibilities (although it does not exclude the possibility of liver disease).2
For borderline isolated LFT abnormalities (i.e. changes in individual markers < 2 times the ULN) in asymptomatic patients, it is reasonable to deliver general liver health advice (e.g. reducing alcohol intake, healthy eating and exercise, if applicable) and re-test LFTs within the next three months (Figure 2). However, more significant changes in individual markers or a progressive increase from previous results may prompt more immediate investigation.
Figure 2 details some causes of isolated marker increases and potential further actions. As an example, GGT is a very sensitive marker, and isolated mild increases commonly occur due to alcohol consumption or medicine use; it does not usually imply significant liver damage/disease unless the increase is marked. Likewise, given that GGT and ALP levels often correlate in the context of liver impairment, isolated increases in ALP may be associated with deficiencies of bone origin rather than liver damage, and therefore fractionated isoenzyme testing may be indicated for persistent elevations, e.g. longer than six months (this may require secondary care referral if not routinely available in local community laboratory).2, 9
In rare instances, enzymes tested for in LFT panels may form macro-complexes with immunoglobulins (e.g. AST with IgA), which reduces renal clearance and leads to elevated results not associated with liver disease.27 Evaluation for LFT macro-complexes can usually be arranged with community laboratories, and this possibility could be considered in patients with persistently elevated individual marker levels with no identifiable cause.
While most liver problems can be managed in primary care, referral for acute hepatology assessment is recommended in all patients with:2, 8
- Any clinical, laboratory or imaging evidence of significant fibrosis or cirrhosis
- Any evidence of acute or chronic synthetic liver failure
- High suspicion of malignancy, e.g. weight loss and marked cholestasis
Non-acute hepatology referral should be considered in:2, 8
- Patients with persistent and unexplainable abnormal LFT results, who have negative serology results and who lack risk factors for MASLD or alcohol-related liver disease*
- Patients with suspected MASLD who have an elevated FIB 4 score (≥ 1.3 if aged ≤ 60 years or ≥ 2.0 if aged > 60 years) AND a FibroScan reading > 8 KPa (see: “FibroScan is an emerging alternative tool to liver biopsy”), as this indicates they are likely to have progressed to steatohepatitis (MASH) and should be referred for management
- Patients with hepatitis B who have an elevated ALT level for at least three months† (N.B. this referral criterion was previously six months)
- Patients with hepatitis C if HCV RNA or antigen is still detectable four weeks after Maviret treatment (N.B. the referral criterion was previously 12 weeks post-treatment) or if they have cirrhosis†
- Patients with hereditary haemochromatosis (i.e. HFE gene mutation) and abnormal LFT findings, hepatomegaly or ferritin > 1,000 micrograms/L**
*For patients with suspected MASLD and alcohol-related liver disease without suspicion of advanced fibrosis, it is reasonable to undertake management and ongoing monitoring in primary care without referring to secondary care.8 See the corresponding sections in “A spotlight on the key causes of liver disease” for more information.
†For further information on:
**For further information on hereditary haemochromatosis, see: https://bpac.org.nz/BT/2015/April/haemochromatosis.aspx
When should ultrasound be requested?
One of the more challenging decisions when investigating abnormal LFT results is deciding if and when an abdominal ultrasound should be requested in patients not being referred for gastroenterology review. Referral for community-based ultrasound is generally indicated in patients with abnormal LFT results and:25, 28, 29
- Suspected cholestasis (i.e. predominantly raised ALP/GGT) or with jaundice where intra- or extra-hepatic obstruction is suspected
- Persistently elevated aminotransferase levels which cannot be explained by first tier investigations (Table 2)
- Suspected gallstone or pancreatic disease, e.g. associated with persistent/recurrent right upper quadrant pain
- Clinical hepatomegaly
- Who are at risk of metastatic liver cancer
- Who require screening for primary hepatocellular carcinoma due to existing cirrhosis
FibroScan is an emerging alternative tool to liver biopsy
Referral for consideration of liver biopsy is sometimes indicated as the next step in patients with suspected liver disease but the diagnosis, prognosis and optimal management approach remains uncertain based on laboratory tests, physical examination and ultrasound.8 However, biopsy is an invasive procedure that has several limitations, e.g. the potential for sampling error, risk of complications and service availability.8
FibroScan is an emerging non-invasive tool in the liver diagnostic/prognostic toolkit, that uses transient elastography to assess the stiffness of the liver which is an indirect measurement of liver fibrosis. FibroScan can diagnose the presence of severe fibrosis or cirrhosis of the liver but it cannot diagnose the underlying cause of the liver disease.30, 31
- Intended as a complement to conventional ultrasound (if required) and not a replacement. FibroScan does not inform on all aspects of structural liver integrity, and ultrasound permits assessment of other features, such as portal hypertension, abdominal varices, recanalisation of the umbilical vein and splenomegaly.30
-
Indications. FibroScan is recommended before initiating Hepatitis C treatment in primary care.* FibroScan is also indicated whenever there is clinical suspicion of advanced fibrosis/cirrhosis, regardless of the underlying cause.30 Use of a relevant clinical scoring tool is often a pre-requisite to access, e.g. NFS, FIB-4 or APRI.
*If FibroScan cannot be accessed, it is acceptable to calculate the patient’s APRI score to estimate fibrosis risk before initiating treatment (see: “A spotlight on the key causes of liver disease”). For further information, see: bpac.org.nz/2019/hepc/pre-treatment.aspx
- Availability and referral criteria vary throughout the country. Clinicians are advised to contact their local gastroenterology service to determine if FibroScan is available in their region, which patients should be referred for assessment and how this is done. In some areas, direct general practitioner referral is available for patients with Hepatitis C infection.
Read more about transient elastography
Transient elastography involves measuring the velocity of vibration waves (“shear waves”) produced by a device positioned on the skins surface.30 By determining the time taken for waves to travel to a particular depth within the liver, stiffness caused by fibrosis or cirrhosis can be determined.30 This information can be used to:30
- Estimate the current level of scarring-associated liver damage
- Detect disease progression or regression if serial measurements are performed
- Support management decisions and guide prognosis
Over-estimation of fibrosis is possible in some cases, including when patients have prominent liver inflammation, liver congestion (right heart failure, tricuspid regurgitation, fluid overload from renal failure) cholestasis or malignancy.30 In addition, accuracy may be reduced in patients with a high BMI (> 30 – 35 kg/m2) or older age.30 FibroScan is usually unsuccessful (i.e. no readings can be obtained) in patients with ascites, or with morbid obesity.
Intrahepatic cholestasis of pregnancy – while elevated ALP levels are common during pregnancy due to an influx of placental enzymes, transient LFT results indicative of cholestasis (i.e. both ALP and GGT increases) may also sometimes occur, particularly during the third trimester. This is referred to as intrahepatic cholestasis of pregnancy (ICP) and the key symptom is pruritus, usually without a rash. The clinical significance of ICP mostly relates to the potential for foetal risk, e.g. pre-term birth, intrauterine death. If a pregnant female reports pruritus but has normal LFT findings, LFTs should be repeated every 10 – 14 days while symptoms persist. If LFT results are abnormal, arrange a liver ultrasound and seek acute obstetric advice (early induction of labour may be considered).
Hyperemesis gravidarum – typically occurs during the first trimester and involves nausea and intractable vomiting (more severe than usual “morning sickness”), resulting in dehydration, ketosis and weight loss. While this is not a true liver disease per se, abnormal transaminase findings occur in approximately half of patients with this condition. For further information on management, see: bpac.org.nz/BPJ/2011/november/pregnancy.aspx
Pre-eclampsia – defined as new onset hypertension occurring after 20 weeks of pregnancy, or when there is pre-existing hypertension and new features of proteinuria, maternal organ dysfunction or uteroplacental dysfunction develop. LFT abnormalities occur in 10% of females with pre-eclampsia and may include mild increases in aminotransferase levels.
HELLP syndrome – a variant of pre-eclampsia which usually occurs in the later stages of pregnancy (or soon after childbirth) and is characterised by haemolysis, elevated liver enzyme levels (usually aminotransferase; typically more severe increases compared with pre-eclampsia) and low platelet counts (< 100 × 109/L). Increased blood pressure and proteinuria may also be present. Risk factors include multiparity and advanced maternal age, and females typically present with fluctuating abdominal pain and non-specific symptoms, e.g. nausea and vomiting.
Acute fatty liver of pregnancy – a rare condition that generally occurs in the third trimester and is characterised by excessive accumulation of fat in the liver. LFT and laboratory marker changes include elevated aminotransferase levels (ranging from mild to > 1,000 IU/L), hyperbilirubinaemia and prolonged INR, which is sometimes accompanied by findings such as leukocytosis, thrombocytopaenia and normochromic anaemia. Potential clinical features include abdominal pain, ascites, pleural effusions, acute pancreatitis and sometimes renal failure. If this condition occurs, acute liver failure and death (of both the mother and baby) is likely unless delivery occurs promptly. The recurrence risk in subsequent pregnancies is high (40 – 70%).
Excluding ICP and hyperemesis gravidarum, all other conditions listed are considered medical emergencies; if suspected, patients require urgent obstetric/secondary care referral
For further information on MASLD, alcohol-related liver disease and hepatitis B- and C-related liver disease, see: “A spotlight on the key causes of chronic liver disease”.





