MASLD is a term describing a spectrum of conditions, ranging from simple steatosis through to inflammation (metabolic dysfunction-associated steatohepatitis [MASH]) and ultimately fibrosis/cirrhosis.1 This was formerly known as non-alcoholic fatty liver disease (NAFLD); the terminology first shifted to emphasise the central role metabolic dysfunction has in disease progression (metabolic dysfunction-associated fatty liver disease; MAFLD), before finally changing to MASLD (pronounced 'ma-zuld') to avoid stigmatisation associated with the term “fatty”. The hallmark feature is excessive fat deposits in the liver in patients with cardiometabolic risk factors, including obesity, hyperglycaemia or insulin resistance, hypertension and hyperlipidaemia (either high cholesterol or high triglycerides).1* The presence of at least one of these risk factors in someone with abnormal LFT results should prompt clinical suspicion that MASLD may be the underlying cause.1 Although there is no specific LFT pattern associated with MASLD, modest increases in aminotransferase levels are common, with ALT being higher than AST (i.e. an AST:ALT ratio < 1).2 MASLD is the most common liver disorder worldwide, affecting approximately one in four people.1
* This nomenclature change was announced in June, 2023, by The American Association for the Study of Liver Diseases (AASLD). The thresholds for cardiometabolic risk factor categories differ between adults and children. If an additional cause of hepatic steatosis is identified outside of a confirmed cardiometabolic driver, the patient is labelled as having a combination aetiology, e.g. MetALD in the case of alcohol, which is distinct from alcohol-related liver disease.
For further information, including specific criteria for cardiometabolic risk factors, see: Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023; Published online ahead of print. doi:10.1097/HEP.0000000000000520
Additional investigations to clarify the clinical picture in patients with abnormal LFTs and risk factors for MASLD include full blood count, HbA1c and fasting lipids. A scoring tool such as the fibrosis-4 (FIB-4) score* or the NAFLD Fibrosis Score (NFS) can be used to stratify patients at risk of fibrosis, and to guide the need for further investigation or referral (Table 1).3, 4 Referral thresholds may differ by region; Figure 1 demonstrates the new secondary referral criteria in the Northern Region Health Pathway (as of October, 2022). There is no consensus concerning the use of liver ultrasound for diagnosing MASLD; while it can be sensitive for detecting fatty liver in severe cases, many patients with simple steatosis appear normal when scanned.1
Table 1. Scoring tools for assessing the risk of fibrosis in patients with suspected MASLD.4
| Scoring tool |
Variables included |
Interpretation of results |
Notes |
Fibrosis-4 (FIB-4) score*
www.mdcalc.com/calc/2200/fibrosis-4-fib-4-index-liver-fibrosis
|
Age, ALT and AST levels, platelet count |
- Thresholds separating fibrosis risk levels may differ depending on the calculator used, regional guidelines and by patient age
- See Figure 1 for an example of risk/referral thresholds
|
May potentially overestimate fibrosis in patients with excessive alcohol intake. Seek gastroenterology advice if score is ≥ 1.3 for people aged ≤ 60 years or score is ≥ 2.0 for people aged ≤ 60 years (or refer for FibroScan, if available).† |
NAFLD Fibrosis Score (NFS)
https://www.mdcalc.com/calc/3081/nafld-non-alcoholic-fatty-liver-disease-fibrosis-score |
Age, BMI, fasting glucose/diabetes status, AST:ALT ratio, platelet count and albumin |
- < -1.455 suggests no significant fibrosis
- –1.455 to 0.675 is an intermediate score
- > 0.675 suggests fibrosis
|
The significance of BMI may differ between ethnic groups. Seek gastroenterology advice if score is ≥ -1.455 (or refer for FibroScan, if available).† |
*An estimate of fibrosis stage (based on Ishak fibrosis staging) is usually given alongside the FIB-4 score.
†Availability and referral criteria may vary throughout the country. Clinicians are advised to contact their local gastroenterology service to determine if FibroScan is available in their region and how patients can be referred for assessment.
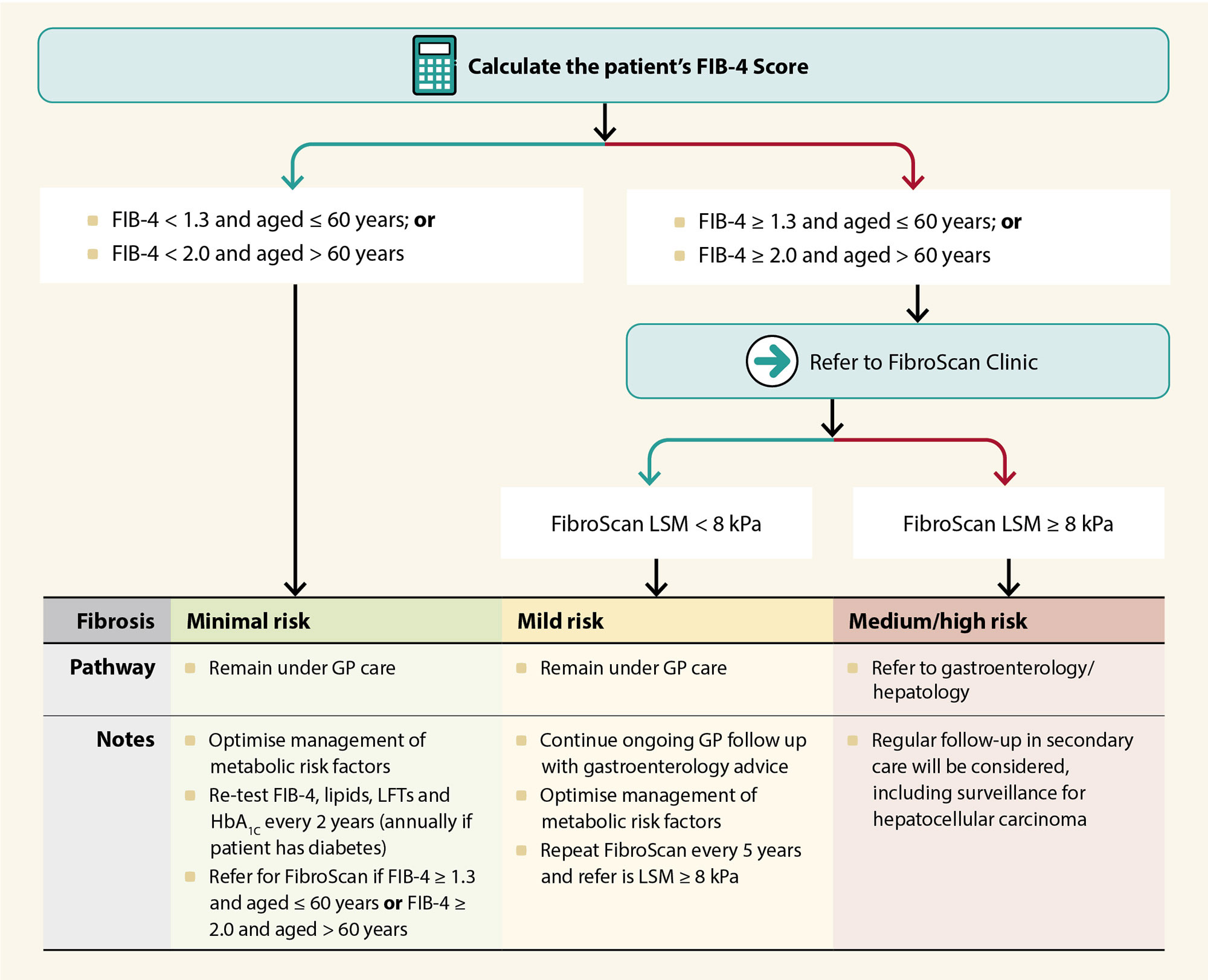
Figure 1. Secondary care referral criteria for the management of MASLD in the Northern Region (NZ), as of October, 2022. N.B. This is an example of a referral pathway only. Applicability to other New Zealand regions will depend on FibroScan availability. For further information on FibroScan, see: “Liver function testing in primary care”. Seek gastroenterology or hepatology advice if there is any uncertainty regarding local referral criteria.
FIB-4, Fibrosis-4; GP, general practitioner; LFTs, liver function tests; LSM, liver stiffness measure
Management. In addition to optimising the management of co-morbidities, the first-line treatment for MASLD is weight loss; ideally involving loss of 7 – 10% of body weight achieved through a combination of healthy eating and exercise:1
- The type of exercise should be tailored to the patient’s capabilities and preference; aerobic, resistance or modified high-intensity interval training have all been demonstrated to reduce liver fat content, potentially via increased tissue blood flow and fat mobilisation
- There is no consensus on the best diet for managing MASLD and several have demonstrated efficacy in clinical trials, e.g. low carbohydrate diets (including ketogenic) and Mediterranean diets. Regardless of the choice, reducing total energy intake is the most important factor. Abstaining from alcohol consumption should also be considered to avoid additional liver damage and fat accumulation, if relevant.
While this approach is desirable in theory, it can be difficult for patients to consistently implement and maintain the necessary challenges.1 For patients unable to implement sufficient lifestyle changes, pharmacological intervention may be considered to assist with weight loss; as of October, 2022, four medicines have been approved for weight loss in New Zealand (none funded).1 Internationally, several medicines are currently being assessed in clinical trials specifically for the treatment of MASLD (i.e. not weight loss). Patients with MASLD and progressively deteriorating liver function despite weight loss interventions should be referred for gastroenterology assessment, where more intensive management may be considered, e.g. bariatric surgery.1
For further information on interventions for weight loss, including pharmacological options, see: https://bpac.org.nz/2022/weight-loss.aspx
Alcohol-related liver disease encompasses a spectrum of liver damage, including alcoholic hepatitis, alcohol-associated steatosis, alcoholic-steatohepatitis and alcoholic-cirrhosis. Excessive alcohol intake is the most common cause of cirrhosis and underpins approximately 75% of liver-related deaths.3, 5 There is an exponential relationship between the level of alcohol consumption and the risk of liver cirrhosis.3
Additional investigations. Any patient with abnormal LFT findings should be asked questions regarding their alcohol intake. It is estimated that one-third of patients with alcohol-related liver disease have severe alcohol dependency or alcoholism.5 If the AST:ALT ratio is > 2, clinicians should be highly suspicious of an alcohol-related liver injury as the cause, particularly if GGT levels are also increased, e.g. > 2 times the ULN.6 However, aminotransferase levels in alcoholic hepatitis are usually only moderately elevated, with AST rarely exceeding 8 – 10 times the ULN and ALT usually < 5 times the ULN.6
Practice point: Not all patients feel comfortable disclosing information relating to alcohol intake and many under-report their level of consumption. The following resources may assist in assessing alcohol misuse in primary care:
Management. As a completely modifiable risk factor, the primary objective for patients with suspected alcohol-related liver disease is to stop them drinking harmfully, ideally through abstinence, if possible.3 Evidence suggests that people who drink excessively would consider changing their behaviour if they were advised by a general practitioner that their drinking was negatively affecting their health.7 In addition:3
- Consider referring any adult with evidence/suspicion of alcohol dependency (i.e. an AUDIT score of ≥ 20) to Community Alcohol and Drug Services (CADS)
- Consider referring any adult with evidence/suspicion of advanced liver disease/cirrhosis for gastroenterology assessment. Given that overt symptoms/signs are not always present in patients with advanced liver disease, a recommended referral threshold is alcohol consumption of ≥ 50 standard drinks per week for males or ≥ 35 standard drinks per week for females.
For further discussion on alcohol misuse, see: https://bpac.org.nz/2018/alcohol.aspx
Infection with hepatitis B or C virus (HBV or HCV) are the most common causes of viral hepatitis. The Hepatitis Foundation estimates that 100,000 people in New Zealand are infected with HBV, and 50,000 people are infected with HCV.8 In both cases, chronic infection can induce liver inflammation and damage, which if left untreated, may progress to more severe liver disease. However, given the lack of specific symptoms, most people with chronic HCV or HBV infection are unaware they are infected until they develop complications of advanced liver disease.9, 10
The majority of adults with chronic HBV were infected at birth or in early childhood, where the risk of developing long-term infection is > 90%.9 In contrast, acute HBV infection in adults usually results in icteric hepatitis followed by spontaneous clearance and lifelong immunity. Most HCV infections occur during adulthood and are usually subclinical, but three-quarters will develop a long-term infection.10
Most patients with viral hepatitis-induced liver damage are identified via targeted
testing based on risk factors, rather than the presence of symptoms:3, 11, 12
| Risk factors for HBV infection: |
Risk factors for HCV infection: |
- Incomplete or unknown childhood vaccination status, particularly in people of Māori, Pacific, Southeast Asian or Chinese ethnicity*
- Age over 30 years*
- People born in countries with a high HBV prevalence, e.g. Pacific Islands, China, South East Asia, Middle East and Africa, or travellers to those countries
- Mother has HBV infection
- Current or previous injecting drug user
- Chronic liver disease or incidental abnormal liver function tests
- Tattoo, piercing or other cosmetic procedure received using unsterile equipment, e.g. in prison, in locations with few safety standards
- Unprotected sex with a HBV-infected person
- Higher risk sexual activity, such as sex workers, men who have sex with men or unprotected sex while travelling in a country with high prevalence
- Following exposure to blood, e.g. sports, assault, needlestick injury
*In addition to other risk factors from the list |
- Current or previous injecting drug user
- Receiving a blood transfusion in New Zealand prior to July, 1992
- People born in countries with a high HCV prevalence, e.g. Eastern Europe, Russia, Egypt and North Africa, India, Pakistan, or travellers to those countries
- Time spent in prison
- A tattoo, body piercing or alteration, e.g. scarification, which was not performed in a licenced premises within New Zealand, i.e. either performed in prison or in a country with a high prevalence of HCV
- History of acute hepatitis, jaundice, or abnormal liver function
- Mother has HCV infection
|
Potential LFT changes. While acute viral hepatitis can induce significant aminotransferase increases (e.g. > 500 U/L), patients with chronic infection generally do not have marked elevations; they are usually < 100 U/L or even within reference range, and the AST:ALT ratio is typically close to 1.6, 13
Additional investigations. Given this ambiguous LFT profile, screening for HBsAg and anti-HCVAb (with follow-up PCR or antigen assessment to confirm infection, if positive) are recommended as routine investigations in most patients with abnormal LFT findings.11 For patients with confirmed chronic HCV infection, the AST to Platelet Ratio Index (APRI; Table 2) can be used to assess their risk of cirrhosis and to guide treatment decisions.11 If viral hepatitis is suspected but testing for Hepatitis B and C is negative, investigation for other potential viral causes should be considered, e.g. hepatitis A, Epstein-Barr virus, Cytomegalovirus.
Table 2. Scoring tool for assessing the risk of cirrhosis in patients with viral hepatitis.11
| Scoring tool |
Variables included |
Interpretation of results |
Notes |
AST to Platelet Ratio Index (APRI)
www.hepatitisc.uw.edu/page/clinical-calculators/apri |
AST level, platelet count |
- < 1.0 indicates no cirrhosis – suitable to proceed with Hep C treatment in primary care, if applicable
- > 1.0 indicates possible cirrhosis (approximately 50% of patients with a score > 1.0 have cirrhosis)
|
Not suitable for assessing cirrhosis risk in patients with acute hepatitis as increased AST is common. Seek gastroenterology advice or refer for FibroScan* if score is > 1.0 and it is available. |
*Availability varies throughout the country. Clinicians are advised to contact their local gastroenterology service to determine if FibroScan is available in their region and how patients can be referred for assessment.
If viral hepatitis is confirmed, management should then be initiated according to the underlying virus. For further information on:





