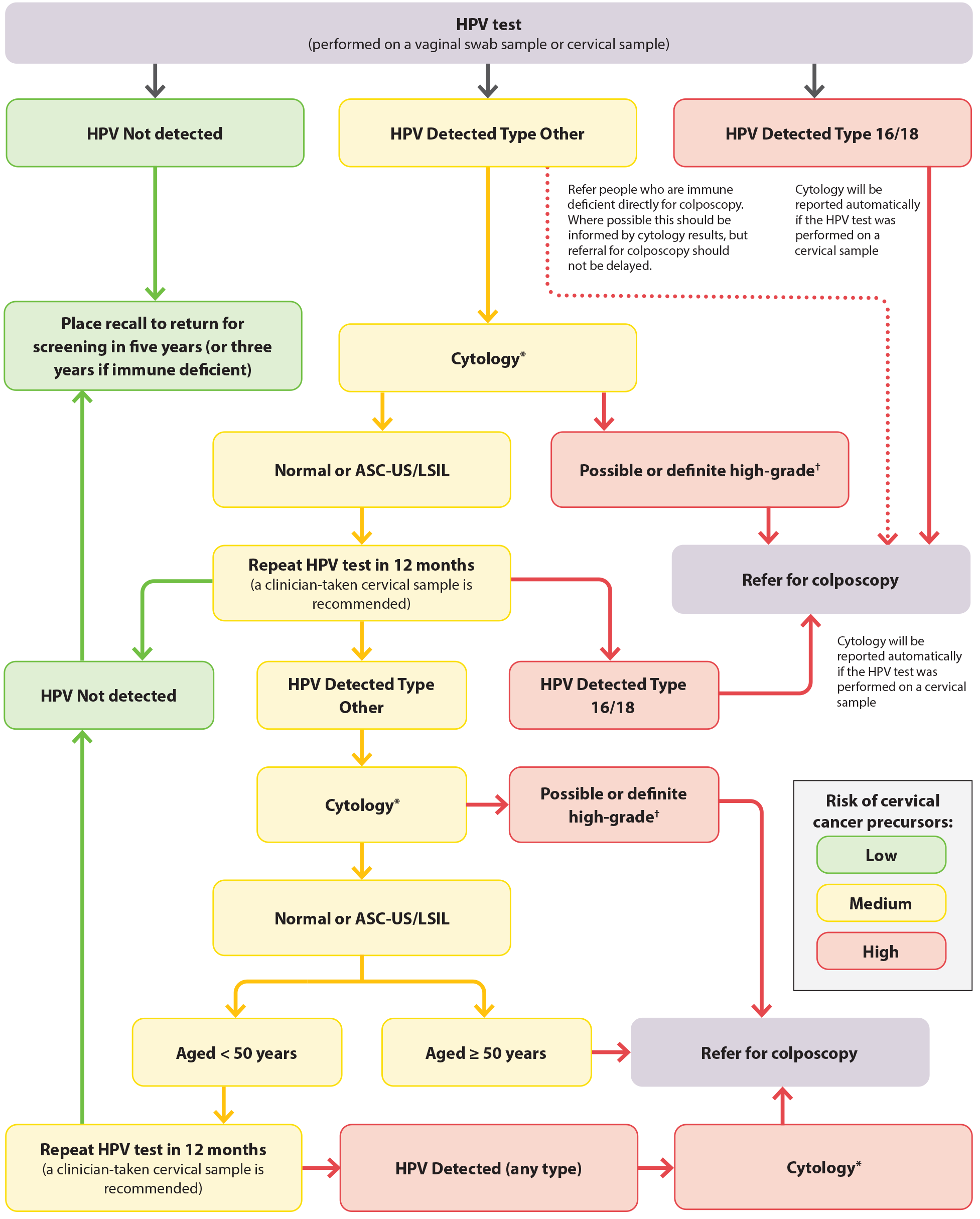
*Cytology cannot be performed from a vaginal swab sample. If the HPV test was conducted from a vaginal swab sample, a return visit is required for a clinician-taken cervical LBC sample with speculum examination.
†Possible or definite high-grade cytology includes ASC-H, HSIL, SCC, atypical glandular cells, adenocarcinoma and AIS
Figure 5. HPV testing for females who are asymptomatic in New Zealand. Adapted from Clinical Practice Guidelines for Cervical Screening in Aotearoa New Zealand, 2023.25
ASC-US = atypical squamous cells of undetermined significance; ASC-H = atypical squamous cells of undetermined significance cannot exclude HSIL; HSIL = high-grade squamous intraepithelial lesion; LSIL = low-grade squamous intraepithelial lesion; SCC = squamous cell carcinoma; AIS = adenocarcinoma in situ
