Anti-inflammatory reliever (AIR) therapy with budesonide/formoterol is now established as the preferred treatment option for adolescents* and adults with asthma. The purpose of this audit is to help health professionals in primary care ensure that all adolescent and adult patients with asthma are being prescribed AIR therapy, unless it is not clinically appropriate. The audit also aims to ensure that AIR therapy has been prescribed correctly, i.e. patients are not prescribed a concurrent SABA reliever, and that no patients aged ≥ 12 years remain on SABA-only treatment, i.e. without maintenance ICS.
* People aged 12 – 17 years; the use of a SABA reliever continues to be recommended in most children aged < 12 years
Historically, short-acting beta2-agonist (SABA) medicines were used as first-line treatments for asthma. However, increasing evidence has associated SABA-only treatment with an increased risk
of exacerbations due to inflammation, sensitivity to allergens and tachyphylaxis. As a result, the 2020 update of the Asthma and Respiratory Foundation NZ Adolescent & Adult Asthma Guidelines advise
against the use of SABA-only treatment in adolescents and adults, instead recommending budesonide/formoterol as first-line treatment for all patients with asthma, including those with mild asthma or
exercise-induced symptoms.
The use of budesonide/formoterol for symptomatic relief (in place of a SABA) is termed “anti-inflammatory reliever” (AIR) therapy. AIR therapy can be used in two ways:
- As needed for symptomatic relief
- As a daily maintenance treatment, with additional doses taken as required for symptomatic relief, i.e. one inhaler is used for both maintenance and relief
When budesonide/formoterol is used for both maintenance and symptomatic relief, this is termed “single combination ICS/LABA inhaler maintenance and reliever therapy”, or SMART.
AIR therapy follows a stepwise progression (see Figure 1). Patients can be switched immediately to AIR therapy from using an as-needed SABA reliever; step 1 of AIR therapy is appropriate for patients with good control previously taking a SABA alone and involves replacing their SABA reliever with budesonide/formoterol. Patients can then step up or down as required to manage their symptoms and reduce exacerbation risk. Patients taking AIR therapy should not be prescribed a concurrent SABA inhaler, as budesonide/formoterol is used as a reliever instead of a SABA.
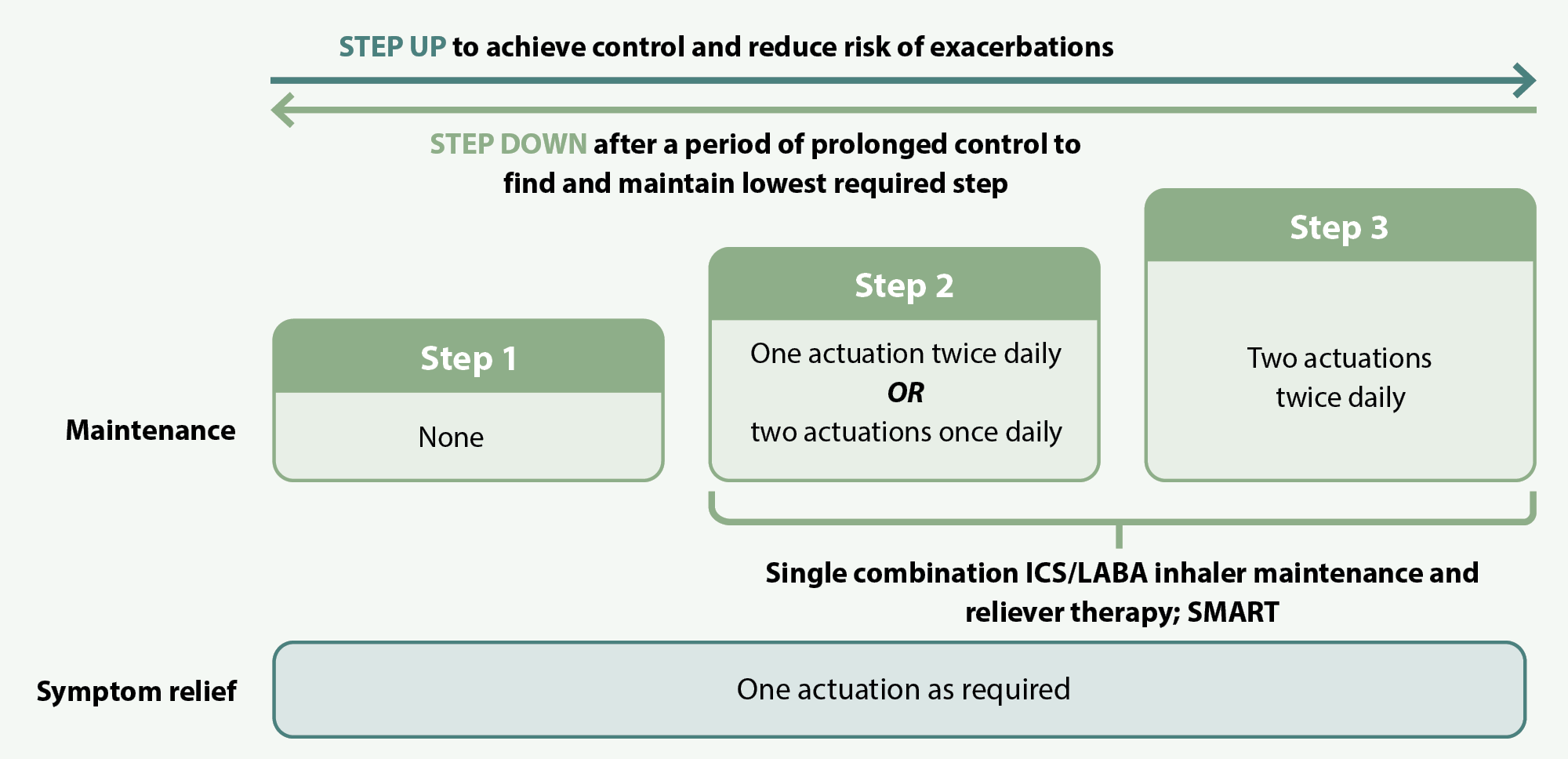
Figure 1. AIR therapy using budesonide/formoterol (200 micrograms + 6 micrograms preferred), adapted from the Asthma and Respiratory Foundation NZ Guidelines (2020).
Treatment of asthma with a SABA alone is no longer recommended. The alternative to AIR therapy is to take an inhaled corticosteroid (ICS) every day for maintenance treatment, with a SABA reliever as needed. This approach is not as beneficial as AIR therapy and is only appropriate for patients who cannot, or do not want to, switch to AIR therapy and are likely to be adherent to daily ICS treatment. People who experience symptoms less than twice per month are often non-adherent to ICS treatment which increases their risk of adverse effects due to SABA overuse. For this reason, AIR therapy (which eliminates the need for a SABA reliever) is preferred, unless patient-specific characteristics prevent this change being practical.
The previous version of this audit focused on patients prescribed a SABA alone, i.e. without maintenance ICS treatment, as they are most at risk of SABA-related adverse effects and therefore will benefit most from being switched to AIR therapy. However, as the guidelines have now been in place since 2020, this revised update focuses on optimising asthma pharmacological control, ensuring that all adolescent and adult patients with asthma are being prescribed AIR therapy, i.e. budesonide/formoterol (alone or in combination with maintenance doses; SMART) instead of a SABA reliever unless clinical justification exists supporting their continued use of a SABA reliever with maintenance ICS treatment.
Summary
This audit helps health professionals in primary care identify adolescent and adult patients with asthma who are not currently prescribed AIR therapy, i.e. budesonide/formoterol, and may benefit from a switch (either alone or in combination with maintenance doses; SMART). It also aims to ensure that these patients are not being prescribed a SABA reliever alone or in combination with AIR therapy.
Recommended audit standards
Ideally, all patients aged ≥ 12 years with asthma will be taking AIR therapy (i.e. a budesonide/formoterol inhaler, either as a reliever alone, or in combination with maintenance doses; SMART) unless clinical justification exists supporting their continued use of a maintenance ICS with a SABA reliever (i.e. two inhalers). Patients should also be reviewed to ensure that they have not been prescribed a SABA reliever alone, i.e. without maintenance ICS treatment, or concurrently with AIR therapy.
 Alternatively, consider a “working audit” where the data sheet is completed over time when any eligible patient presents. Document whether they are currently prescribed AIR therapy, and if not whether there is clinical justification for their continued use of a maintenance ICS with a SABA reliever. Patients for whom there is no justification or who have been inappropriately prescribed a SABA reliever should be flagged for review.
Alternatively, consider a “working audit” where the data sheet is completed over time when any eligible patient presents. Document whether they are currently prescribed AIR therapy, and if not whether there is clinical justification for their continued use of a maintenance ICS with a SABA reliever. Patients for whom there is no justification or who have been inappropriately prescribed a SABA reliever should be flagged for review.
Eligible patients
Any patients aged ≥ 12 years with a diagnosis of asthma.
Identifying patients
You will need to have a system in place that allows you to identify eligible patients and audit their clinical notes. Many practices will be able to do this by running a “query” through their PMS to find patients aged ≥ 12 years with a diagnosis of asthma.
If conducting a working audit, fill in the data sheet when you have a consultation for any reason with a patient aged ≥ 12 years with asthma over the course of an allocated time frame.
Sample size
The number of eligible patients will vary according to your practice demographic. If a large number of results are returned, a sample size of 20 – 30 patients is sufficient for this audit. However, all eligible patients should be reviewed subsequently over time.
If conducting a working audit, a smaller sample size may be necessary to complete it within your planned time frame.
Criteria for a positive outcome
A positive result is any patient with asthma who has already been prescribed AIR therapy, i.e. budesonide/formoterol (either alone or in combination with maintenance doses; SMART) instead of a SABA reliever, or has not been prescribed AIR therapy but has adequate clinical justification to support their continued use of a SABA reliever with maintenance ICS treatment instead.
Patients taking AIR therapy with a concurrent prescription for a SABA should not be considered a positive result. Some patients who had previously used a SABA reliever for asthma control long-term may have been reluctant to switch to AIR therapy initially, resulting in the prescription of a concurrent SABA inhaler for reassurance. However, this is not best practice; patients taking AIR therapy should not also be prescribed a SABA, as budesonide/formoterol is used as a reliever instead of a SABA. Patients prescribed a SABA alone, i.e. without ICS treatment, should also not be considered a positive result.
If conducting a working audit, and the patient is not currently prescribed AIR therapy or is using a SABA reliever alone or in combination with AIR therapy, a review should be undertaken at the time, or a future appointment booked to review their treatment.
Data analysis
Use the sheet provided to record your data. The percentage achievement can be calculated by dividing the total number of patients with a positive result (sum of ‘YES’ responses in both columns) by the total number of patients audited.
Clinical audits can be an important tool to identify where gaps exist between expected and actual performance. Once completed, they can provide ideas on how to change practice and improve patient outcomes. General practitioners are encouraged to discuss the suitability and relevance of their proposed audit with their practice or peer group prior to commencement to ensure the relevance of the audit. Outcomes of the audit should also be discussed with the practice or peer group; this may be recorded as a learning activity reflection if suitable.
The Plan, Do, Study, Act (PDSA) model is recommended by the Royal New Zealand College of General Practitioners (RNZCGP) as a framework for assessing whether a clinical audit is relevant to your practice. This model has been widely used in healthcare settings since 2000. It consists of two parts, the framework and the PDSA cycle itself, as shown in Figure 2.
1. The framework
This consists of three questions that help define the “what” and “how” of an improvement project (in this case an audit).
The questions are:
- "What are we trying to accomplish?" – the aim
- "How will we know that a change is an improvement?" – what measures of success will be used?
- "What changes can we make that will result in improvement?" – the concept to be tested
2. The PDSA cycle
This is often referred to as the “engine” for creating, testing and carrying out the proposed changes. More than one cycle is usually required; each one is intended to be short, rapid and frequent, with the results used to inform and refine the next. This allows an ongoing process of continuous learning and improvement.
Each PDSA cycle includes four stages:
- Plan – decide what the change to be tested is and how this will be done
- Do – carry out the plan and collect the data
- Study – analyse the data, assess the impact of the change and reflect on what was learned
- Act – plan the next cycle or implement the changes from your plan
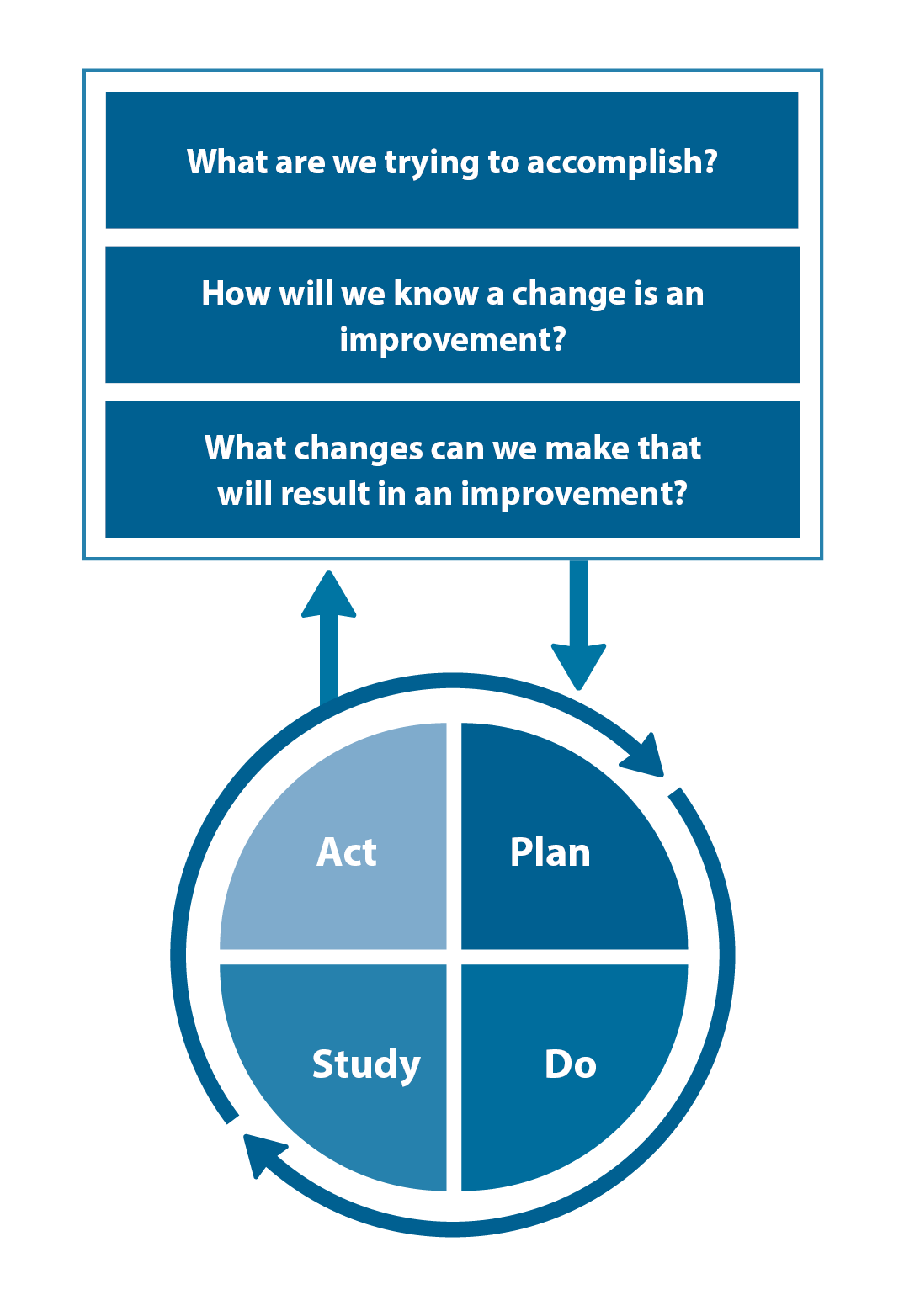
Figure 2. The PDSA model for improvement.
Source: Plan, Do, Study, Act (PDSA) cycles and the model for improvement

Claiming credits for Te Whanake CPD programme requirements
Practice or clinical audits are useful tools for improving clinical practice and credits can be claimed towards the Patient Outcomes (Improving Patient Care and Health Outcomes) learning category of the Te Whanake CPD programme, on a two credit per learning hour basis. A minimum of 12 credits is required in the Patient Outcomes category over a triennium (three years).
Any data driven activity that assesses the outcomes and quality of general practice work can be used to gain credits in the Patient Outcomes learning category. Under the refreshed Te Whanake CPD programme, audits are not compulsory and the RNZCGP also no longer requires that clinical audits are approved prior to use. The college recommends the PDSA format for developing and checking the relevance of a clinical audit.
To claim points go to the RNZCGP website: www.rnzcgp.org.nz
If a clinical audit is completed as part of Te Whanake requirements, the RNZCGP continues to encourage that evidence of participation in the audit be attached to your recorded activity. Evidence can include:
- A summary of the data collected
- An Audit of Medical Practice (CQI) Activity summary sheet (Appendix 1 in this audit or available on the
RNZCGP website).
N.B. Audits can also be completed by other health professionals working in primary care (particularly prescribers), if relevant. Check with your accrediting authority as to documentation requirements.





