Ayurveda is a traditional medicine system that originated in India more than 3,000 years ago.1 It is still widely practiced throughout Asia and other parts of the world.1 The term Ayurveda loosely translates to “knowledge (or science) of life”.1, 2 The use of plants, minerals and animal products for health and treatment of disease is an important component of Ayurveda, and they are prescribed based upon principles relating to classical elements, imbalances and essential tissues.1
Rasashastra, a branch of Ayurvedic medicine, is notable for integrating heavy metals into treatments.1 Many of these preparations contain high levels of lead, mercury, arsenic* and cadmium.3 These elements are believed to enhance the effects of other ingredients or have therapeutic properties themselves, e.g. mercury (referred to as Parad in Ayurveda) is used as an anthelmintic, arsenic compounds (e.g. Haritala) are used as antipyretics and to treat sexually transmitted infections.1
The use of heavy metals in treatments is problematic as their toxicity is well established and is associated with adverse effects in multiple organ systems, e.g. brain, heart, liver, kidneys, skin.4 Toxicity primarily occurs due to oxidative stress and disruption of cell signalling pathways and antioxidant defence mechanisms.4 A key component of Rasashastra is the “detoxification” or “purification” of metals before they are incorporated into herbal preparations, but these processes are ineffective at reducing their toxicity.5
* Arsenic is a metalloid with properties of both a metal and a non-metal, but is generally included under the umbrella term “heavy metal”
Lead is the primary concern in Ayurvedic medicines
Lead is the most frequently identified heavy metal in Ayurvedic medicines; a New Zealand study published in 2025 found that two-thirds of the 75 Ayurvedic medicines analysed contained lead (see: “Heavy metal content of Ayurvedic medicines available in New Zealand”).3 Lead exposure is notifiable to the National Public Health Service because it poses a significant, and avoidable, health risk. Lead exposure occurs from a diverse range of both occupational (e.g. construction, manufacturing, mining) and non-occupational sources (e.g. lead-based paint, indoor rifle shooting, hobby manufacturing of ammunition or sinkers).6, 7
Between 2014 and 2023, there were 1,195 notifications of patients with elevated blood lead levels made to the National Public Health Service.7 Of these, 28 exposures were due to traditional medicines or cosmetics.7 The notifiable blood lead level was lowered from ≥ 0.48 micromol/L (10 mg/dL) to ≥ 0.24 micromol/L (5 mg/dL) in April, 2021, accounting for some of the increase in later years, however, a cluster of cases involving Ayurvedic medicines has recently emerged.8
For further information on lead exposure in general, see: https://bpac.org.nz/2021/lead.aspx
Medsafe first published an Alert Communication in March, 2024, following eight notified cases of elevated blood lead levels suspected to involve Ayurvedic medicines since October, 2023.9 As of May, 2025, there have now been 22 notified cases, in which 19 involved people confirmed to be taking Ayurvedic medicines (the remaining three cases were suspected to involve Ayurvedic medicines).8 Most cases in the cluster involved males (77%) in the North Island; half were aged 25 – 34 years.8 Almost all cases were in people of Asian - Indian ethnicity, highlighting the higher use of Ayurvedic medicines in this group compared with the general population.8 The median blood lead level was 3.5 micromol/L, well above the notifiable level, and the highest level exceeded 10 micromol/L.8 Many of the patients were symptomatic, e.g. abdominal pain, nausea, vomiting, constipation, and required hospital-level care.8 Because of the non-specificity of the symptoms (and in some cases patients were asymptomatic), a diagnosis of lead toxicity was not always initially made. N.B. Several cases involved Kamini, an opioid-containing Ayurvedic medicine, which may account for some of the symptoms reported (see: “Patients taking Kamini – do not just worry about opioids”).
The Ayurvedic medicines implicated in these cases were mainly sourced from India directly or brought to New Zealand by family or friends (Figure 1).8 A small number of products were believed to have been purchased in New Zealand.8 The samples that were available for analysis by regulators were described as small, circular, flat tablets, of approximately 7 mm in diameter and black, dark grey or dark brown in colour.9 Many of the products did not have labels, manufacturers details or ingredients listed, making initial identification challenging.8

Figure 1. Samples of Ayurvedic medicines purchased in India and brought into New Zealand in personal luggage. Image provided by the National Public Health Service.
Legal status of Ayurvedic medicines in New Zealand
The legal status of Ayurvedic medicines in New Zealand is ambiguous. Ayurvedic medicines are only governed under the Medicines Act 1981 if they claim to have a therapeutic purpose or contain an ingredient with an accepted therapeutic purpose. No Ayurvedic medicines are approved by Medsafe, but there is no basis to stop people from bringing Ayurvedic medicines into New Zealand for personal use unless they contain a controlled substance. For example, the importation, supply and possession of Kamini, a type of Ayurvedic medicine containing opioids, without a prescription is prohibited in New Zealand under the Misuse of Drugs Act 1975 (see: “Patients taking Kamini – do not just worry about opioids”).
Heavy metal content of Ayurvedic medicines available in New Zealand
In 2024, in response to the increase in notified cases, the New Zealand Institute for Public Health and Forensic Science (PHF Science; formerly known as the Institute of Environmental Science and Research [ESR]) investigated the heavy metal content of Ayurvedic medicines available for purchase from retail stores in Auckland and Christchurch (n = 49) or New Zealand-based online stores (n = 26).3 Of the 75 samples, 58 (77%) had detectable levels of either lead, arsenic, cadmium or mercury.3 As a result, 13 Ayurvedic medicines with high levels of heavy metals were removed from sale in New Zealand due to the potential health risks.3
Results of the analysis:
- Lead found in 50 samples (67%), none above the permissible limit recommended by the World Health Organization and the United States Pharmacopeia
- Mercury found in 17 samples (23%), ten above the permissible limit (of which eight were from the same manufacturer)
- Arsenic found in 26 samples (35%), one above the permissible limit
- Cadmium found in 33 samples (44%), two above the permissible limit
It was noted that the levels of heavy metals detected in these samples available to purchase in New Zealand were lower than those reported from similar testing carried out in other countries, e.g. Italy, United States of America.3 People who source Ayurvedic medicines from outside of New Zealand may be at higher risk of heavy metal toxicity compared to those who purchase products from local retailers. Many of the products implicated in the recent lead poisoning case cluster were sourced from overseas.8
Healthcare professionals should also be aware that there are no manufacturing quality standards in place for Ayurvedic medicines, meaning that there can be substantial variation in lead content between products and within different batches of the same product. Do not assume that an Ayurvedic medicine contains a low risk lead concentration based on previous test results.
Calls to the National Poisons Centre involving heavy metals are increasing
 The New Zealand National Poisons Centre (NPC) has received 59 contacts relating to 36 unique heavy metal exposures involving traditional or herbal medicines and supplements since 2018 (to April, 2025).10 Half of these contacts occurred after 2022.10 Lead was the most common heavy metal involved, accounting for 19 contacts (of which 11 were reported in 2024).10 Ayurvedic medicine was the suspected source of lead in 15 of these contacts.10 Mercury accounted for 16 contacts over this period; many of these involved skin-whitening creams.10 Arsenic accounted for one contact, while there were no enquiries to the NPC regarding cadmium exposure during this period.10 Almost all contacts involved people aged 20 years and over, and more than half of the contacts were received from healthcare professionals, suggesting the people involved had already presented to a medical centre or hospital.10
The New Zealand National Poisons Centre (NPC) has received 59 contacts relating to 36 unique heavy metal exposures involving traditional or herbal medicines and supplements since 2018 (to April, 2025).10 Half of these contacts occurred after 2022.10 Lead was the most common heavy metal involved, accounting for 19 contacts (of which 11 were reported in 2024).10 Ayurvedic medicine was the suspected source of lead in 15 of these contacts.10 Mercury accounted for 16 contacts over this period; many of these involved skin-whitening creams.10 Arsenic accounted for one contact, while there were no enquiries to the NPC regarding cadmium exposure during this period.10 Almost all contacts involved people aged 20 years and over, and more than half of the contacts were received from healthcare professionals, suggesting the people involved had already presented to a medical centre or hospital.10
It is best practice to consult with the NPC if there is any uncertainty regarding the treatment of a patient who has been exposed to a potentially harmful substance. Poisons Information Officers are extensively trained to aid with appropriate advice including decontamination and antidotes, relevant monitoring and overall patient management. A medical toxicologist is available 24-hours a day, seven-days a week to provide in-depth clinical advice in more complex or emergency situations.
 0800 764 766
0800 764 766
For more information about the NPC, visit: poisons.co.nz
It is uncommon for a patient to present with lead toxicity in primary care and diagnosis is challenging as symptoms are insidious, non-specific and involve multiple biological systems. However, given the recent case cluster, primary care clinicians should include lead and other heavy metal exposures in their differential diagnosis for patients presenting with non-specific symptoms and known or suspected use of Ayurvedic medicines.
Symptoms and signs of lead toxicity
Symptoms and signs of lead toxicity vary substantially at presentation.6 Symptoms do not appear immediately after exposure and may not be evident until the person’s blood lead level is > 1.45 micromol/L.11Non-specific gastrointestinal and neurological features are therefore a typical initial presentation, including:12
- Gastrointestinal – reduced appetite, nausea, diarrhoea or constipation, weight loss and abdominal pain
- Neurological – headache, mood disturbance (e.g. irritability or depression), memory impairment and tingling/numbness in the hands/fingers; in severe cases, lead-induced toxicity may cause encephalopathy, presenting as altered mental state (request an emergency assessment in secondary care)
- Systemic – excessive fatigue, decreased libido, sleep disturbances
Other symptoms associated with elevated blood lead levels include hypertension, and renal and hepatic dysfunction.6, 12
For symptoms and signs of toxicity relating to other heavy metals see Table 1; discuss patients with a relevant exposure history with a toxicologist or public health physician.
Table 1. Clinical features of heavy metal toxicity following oral exposure.3, 11–14
|
Environmental sources |
Symptoms and signs of acute exposure |
Symptoms and signs of chronic exposure |
Lead (Pb) |
- Lead-based household paints and paint dust (most common; likely to be present in pre-1980 paintwork and old toys or cots)
- Drinking water from lead pipes (less of a concern in New Zealand) or rainwater collected on roofs or guttering containing lead nails or flashings
- Certain types of machinery containing lead-based components, e.g. car radiators, lead-acid batteries
- Ammunition
- Leaded pottery and ceramic glazes
- Some imported cosmetics (e.g. lipsticks)
- Traditional remedies, including Ayurvedic medicines
|
- Vomiting
- Diarrhoea
- Metallic taste
- Abdominal pain
- Acute kidney injury
- Hepatic failure
- Neurological dysfunction (encephalopathy, seizures, coma)
N.B. Development of symptoms following acute lead exposure is variable, even with high blood lead levels. |
- Anorexia
- Abdominal pain
- Constipation
- Lead lines (or burton lines; bluish/purple discolouration along the gum line)
- Confusion
- Headaches
- Behavioural changes
- Poor concentration
- Peripheral or motor neuropathy
- Encephalopathy
- Anaemia
- Chronic kidney disease
- Hepatic failure
- Infertility
- Reduced libido
|
Mercury (Hg; inorganic) |
- Industrial processes, e.g. mining, coal power plants
- Skin-lightening creams
- Traditional remedies, including Ayurvedic medicines
|
- Vomiting
- Diarrhoea
- Gingivostomatitis (inflammation of oral mucosa and gums)
- Memory loss
- Irritability or depression
- Tremor
- Paraesthesia
- Hypertension
- Flushing, discoloration and desquamation of the hands and feet
|
- Vomiting
- Diarrhoea
- Central nervous system dysfunction
- Mood changes
- Tremors
- Hypertension
- Renal dysfunction
- Skin rashes
|
Arsenic (As; inorganic) |
- Contaminated soil (near gold mines)
- Smoke from treated timber
- Some imported cosmetics
- Food grown in contaminated soil
- Traditional remedies, including Ayurvedic medicines
|
- Vomiting
- Diarrhoea
- Abdominal pain
- Neurological dysfunction (encephalopathy, seizures, coma)
- Cardiac conduction abnormalities (severe cases)
|
- Sensorimotor peripheral neuropathy
- Anaemia
- Pancytopaenia
- Hyperkeratosis affecting predominantly the palms and soles
- Hyperpigmentation (and hypopigmentation) of skin on the face, neck and back
|
Cadmium (Cd) |
- Nickel-cadmium battery manufacture
- Contaminated soil
- Metal work, e.g. steel coating
- Yellow and red pigments
- Food grown in contaminated soil
- Tobacco smoke
- Traditional remedies, including Ayurvedic medicines
|
- Vomiting
- Abdominal pain
- Salivation
- Dizziness
- Seizures
- Cardiogenic shock
- Muscle cramps
|
- Cognition and behavioural changes
- Central nervous system dysfunction
- Proteinuria
- Toxic nephropathy
- Bone demineralisation
- Osteomalacia
- Osteoporosis
|
Spotlight: Heavy metal toxicokinetics
Lead. Lead exposure occurs through ingestion or inhalation via a variety of occupational and non-occupational sources, e.g. lead oxide in paints and pottery glazes, lead chromate in pigments, lead sulfate in lead-acid batteries (see: “Lead is the primary concern in Ayurvedic medicines”).15 Following absorption, almost all serum lead is bound to red blood cells and has a half-life of ~ 30 days.11 Over time, 98% of the total body lead is deposited into skeletal bones. Release of lead back into the blood is slow; the half-life can be up to ten years.11 Approximately two-thirds of lead is excreted in the urine (65%) with the remaining excreted in the faeces; minimal excretion occurs via the hair, nails and sweat.15
Mercury. Mercury exposure may be via inorganic (e.g. mercury salts) or organic (e.g. environmental methylmercury) compounds or elemental mercury, i.e. pure metal liquid or vapour. The features of toxicity are, however, the same, e.g. central nervous system and renal toxicity.11 Mercury exposure relating to traditional medicines generally involves inorganic mercury.11 Inorganic mercury exposure occurs via ingestion and dermal absorption and is excreted in the faeces and urine.11 It has a half-life of one to three weeks in the blood and one to three months in the urine.11 Organic methylmercury accumulates in fish and is well absorbed from the gastrointestinal tract.11 Liquid elemental mercury, found in thermometers, sphygmomanometers and fluorescent light bulbs, typically only poses a risk if the product is damaged and clean-up is delayed or ineffective.11 Liquid mercury is volatile and releases vapour at room temperature; this process can be accelerated by vacuuming.11 Infants crawling on the floor are most likely to be exposed to mercury in this situation.
Arsenic. Arsenic poisoning is rare.11 Exposure occurs via ingestion, inhalation or through dermal absorption.11 Soluble inorganic arsenic found in water, soil and traditional medicines is substantially more toxic than organic arsenic which is commonly found in seafood (arsenobentaine).11 Both red and white blood cells carry absorbed arsenic to the liver and other organs in smaller quantities, e.g. kidneys, muscles, heart, lungs, pancreas, brain.4 Approximately 45 – 70% of absorbed arsenic undergoes biomethylation to dimethylarsinic acid (DMA) and methylarsonic acid (MMA) in the liver.11 The half-life of arsenic is one to two hours in the blood, compared to 30 hours in other tissues.11 It is predominantly eliminated via the kidneys.11
Cadmium. Exposure mainly occurs via inhalation in an occupational setting, e.g. battery manufacture, yellow and red pigments, or ingestion of foods grown in soil contaminated by industry or phosphate fertilisers.16 Absorption of cadmium via the lungs is higher than through the gastrointestinal tract.16 A person’s nutritional status affects cadmium absorption; low iron stores may promote cadmium absorption.16 Cadmium predominantly bioaccumulates in the liver and kidneys.16 Excretion occurs slowly and mainly occurs via the kidneys; the half-life of cadmium ranges from 16 to 30 years.13 Other excretion routes include faeces, saliva and sweat.13
Identifying a potential exposure
Recognising a clear exposure source is essential with suspected lead toxicity to avoid unnecessary investigations and delayed diagnosis. Clinicians should ask about the patient’s use of complementary and alternative medicines (CAMs) as part of a complete history. Patients who use Ayurvedic medicines or have other conventional risk factors for lead exposure, e.g. painting, mining, smelting/metal working, target/indoor rifle shooting, are at higher risk of elevated blood lead levels. The National Public Health Service staff can help with investigating exposure sources, including testing products or medicines.
Best Practice Tip: Getting the full picture
Consider reframing the question if the patient initially denies using complementary products but clinical suspicion remains high. Ayurvedic medicines have a wide range of indications, including sexual dysfunction and infertility.1 Kamini is also illegal to possess without a prescription (see: “Legal status of Ayurvedic medicines in New Zealand”). Some patients may be hesitant to admit their use of traditional medicines due to fear of judgement, embarrassment or perceived legal consequences.17 Clinicians should approach these conversations in an unbiased and non-judgemental way to promote trust in the doctor-patient relationship and help the patient to make informed decisions.17
The Medical Council of New Zealand has published guidance on discussing CAMs with patients, available from: https://www.mcnz.org.nz/our-standards/current-standards/complementary-and-alternative-medicine-cam/
Patients taking Kamini – do not just worry about opioids
In 2020, bpacnz published a case study of ten males treated for opioid dependence relating to Kamini and Barshasha (used in Unani medicine). Kamini (also referred to as Kamini Vidrawan Ras, KVR) is an opioid-containing Ayurvedic medicine and is purported to have a wide range of benefits for male sexual functioning and overall vitality (Figure 2). Doses as high as 24 – 36 pellets per day were reported (the instructed dose is 0.5 – 1 pellet per day), and all ten males required opioid substitution treatment. The key message for primary care clinicians was to assess patients using these products for symptoms and signs of opioid dependence, e.g. ask about any problems when they miss a dose or try to stop.
Considering Kamini preparations were identified in the recent case cluster, lead toxicity may also be relevant in people who use it. Therefore, in addition to assessing for opioid dependence, clinicians should also consider requesting a blood lead level for a patient who presents with a history of Kamini use.

Figure 2. Kamini comes in the form of small pellets.
Arrange relevant investigations
Request a blood lead level if there is clinical suspicion of lead poisoning based on the patient’s history of taking Ayurvedic medicines or symptoms.11
Also consider requesting:
- Full blood count – anaemia is common in people with lead poisoning11
- Renal and liver function testing – persistently elevated blood lead levels are associated with renal and hepatic dysfunction4, 18
Notification of an elevated blood lead level
The local Medical Officer of Health must be notified of any patient with a blood lead level ≥ 0.24 micromol/L (5 mg/dL) under the Health Act 1956 and the Hazardous Substances and New Organisms Act 1996. Notification is required regardless of whether it is an acute, ongoing or past exposure. This can be done using the Hazardous Substances Disease and Injury Reporting Tool (HSDIRT) accessed via your practice management system, or by phoning or emailing a notification form to the local National Public Health Service office (see: “Notifying cases of hazardous substance exposures using HSDIRT”).
Other forms of heavy metal toxicity are not considered notifiable unless environmental contamination is the suspected source. However, given the current concerns, it is appropriate to notify the National Public Health Service of any patient with elevated levels of heavy metals relating to Ayurvedic medicines.
Collect a sample of the Ayurvedic medicine for testing, if possible. The National Public Health Service is asking clinicians to obtain a sample of the Ayurvedic medicine from the patient, where possible, if heavy metal toxicity is suspected. All costs associated with testing are usually covered; contact your local National Public Health Service office for more information.
Public health physicians will provide advice on appropriate management and follow-up once notification of an elevated blood lead level is received.12 This includes arranging for repeat testing, arranging appropriate referrals and ongoing monitoring through serial blood lead measurements (in partnership with the primary care practitioner).12
Chelation treatment, which involves binding of metal ions to enhance elimination, may be required if blood lead levels are ≥ 3.4 micromol/L in adults (or at lower levels in children and pregnant females).12 Chelation treatment disrupts the established equilibrium between lead in the blood and lead stored in bones.11 Redistribution of lead back into the blood after chelation treatment can result in an increase in blood lead levels, but re-exposure should also be considered if this occurs.11 Blood lead levels should ideally be measured immediately before and after treatment, and again one to four weeks later depending on the initial level to determine if further chelation is required.6, 11 A persistently elevated blood lead level may indicate ongoing lead exposure.6
For further information on how patients with elevated blood lead levels are managed by the National Public Health Service, see: The Environmental Case Management of Lead-Exposed Persons, Guidelines for Public Health Officers (2024)
Education for asymptomatic patients with blood lead levels below the notifiable level
Reducing further lead exposure is a critical component of management.11 All patients who are identified as using Ayurvedic medicines should be informed of the potential risks of continued use of these products. Consider whether annual blood lead monitoring is appropriate if the patient does not wish to stop using Ayurvedic medicines. Further information regarding minimising risks should also be provided, e.g. avoiding other sources of lead exposure.
Patients who are found to have notifiable blood lead levels should be advised to stop taking the Ayurvedic medicine. It is recommended that patients who have been using Kamini (or other opioid-containing Ayurvedic medicines) long term should be discussed with a local specialist alcohol and drug service, as abrupt cessation can induce opioid withdrawal; opioid substitution treatment may be required.
Information about non-occupational lead exposure for patients is available from: https://info.health.nz/conditions-treatments/emergencies-and-first-aid/lead-poisoning
Notifying cases of hazardous substance exposures using HSDIRT
By law, injuries and diseases due to hazardous substances (Hazardous Substances and New Organisms Act 1996) must be notified to the Medical Officer of Health.
Environmental Health Intelligence NZ (EHINZ), Massey University, provides a Hazardous Substances Surveillance System (HSSS) for New Zealand. This system monitors diseases, injuries and deaths from hazardous substance exposures. Primary care notifications allow identification of substances that are causing harm and can lead to public health action to prevent disease or injury.
For further information on the HSSS, see: http://www.ehinz.ac.nz/indicators/hazardous-substances/
A hazardous substance is anything that can explode, catch fire, oxidise, corrode or is toxic to humans, as defined in the Hazardous Substances and New Organisms Act 1996.
N.B. This definition does not include pharmaceuticals in finished dose form (i.e. over-the-counter and prescription medicines), alcohol when classified as a food, chemical toxins associated with food, or radioactive materials as these are covered by different legislation. Manufactured articles other than those including substances with explosive properties such as fireworks are also not included, e.g. batteries.
BPAC Clinical Solutions, in association with EHINZ, and funded by Health New Zealand, has developed a Hazardous Substances Disease and Injury Reporting Tool (HSDIRT), that enables electronic reporting of all hazardous substance exposures (Figure 3).20 It is available via Medtech, Indici, MyPractice and Profile practice management systems.20
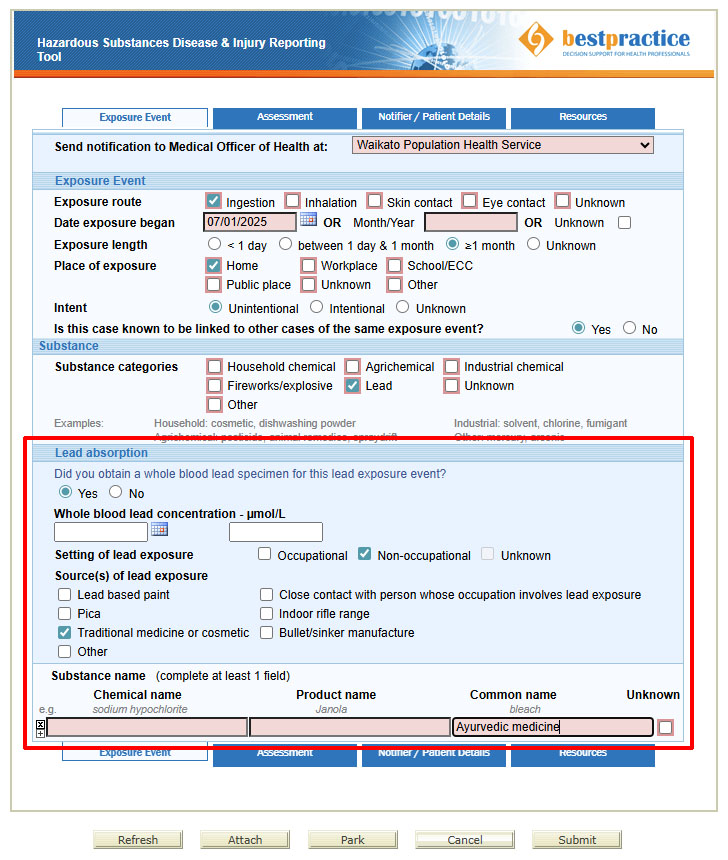
Figure 3. An example of reporting an exposure in the Hazardous Substances Disease and Injury Reporting Tool.
The notification tool will show three tabs for clinicians to complete: “Exposure Event”, “Assessment” and “Notifier/Patient Details” (Figure 3). Submitting the notification will send it to your local Medical Officer of Health via a secure system.
To access HSDIRT, go to the bestpractice Decision Support dashboard in your practice management system (Figure 4):
- Look for “Module List”
- Expand the “Hazardous Substances & Lead Notifications” tab
- Click on “Hazardous Subs & Lead Notifications”
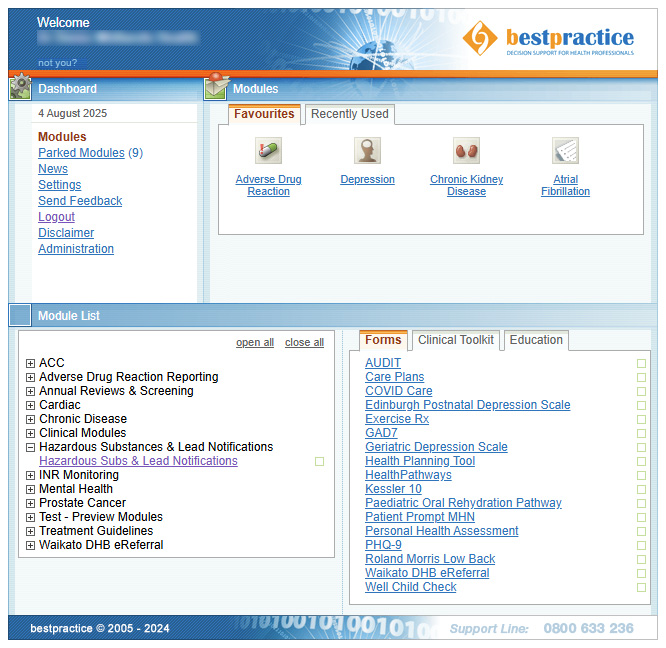
Figure 4. The bestpractice Decision Support dashboard.
If your practice does not have access to this tool and you want to make a notification, phone your local public health service directly. The staff will enter the case information into a HSDIRT notification form on your behalf.20
For a walkthrough video explaining how to access and complete HSDIRT notification forms, see: https://vimeo.com/359445622
To get bestpractice Decision Support software for your practice, contact the BPAC Clinical Solutions Helpdesk:
 0800 633 236
0800 633 236
Email: itservicedesk@southlink.co.nz
Website contact form: https://bpacsolutions.co.nz/contact/
For further information on hazardous substances disease and injury notifications, see: https://bpac.org.nz/BPJ/2016/May/e-notification.aspx
