Quinolones are a class of broad-spectrum antibiotics that inhibit bacterial DNA synthesis. The addition of a fluorine
atom to a quinolone forms a subset of medicines referred to as fluoroquinolones, which have enhanced antimicrobial activity.
Fluoroquinolones available in New Zealand include:1
- Ciprofloxacin (tablets, eye drops, ear drops* and solution for IV infusion)
- Norfloxacin (tablets)
- Moxifloxacin (tablets and solution for IV infusion)
- Levofloxacin (tablets [section 29, unapproved medicine])
*Formulated with hydrocortisone; indicated for the treatment of otitis externa if Pseudomonas is
suspected
N.B. Prescribing restrictions, endorsements and Special Authority criteria apply, see Table 1 for
details.
Quinolones are most active against Gram-negative bacteria
Quinolones are very active against aerobic Gram-negative bacilli and cocci, including Enterobacteriaceae, Pseudomonas
aeruginosa, Haemophilus influenzae, Moraxella catarrhalis (Branhamella catarrhalis) and Neisseria gonorrhoeae
. They are generally less active against Gram-positive organisms such as staphylococci and much less active against streptococci
such as Streptococcus pneumoniae.2 Quinolones are generally not effective against anaerobic organisms.
Moxifloxacin is a later generation quinolone and has greater activity against Gram-positive organisms and atypical organisms
than ciprofloxacin or norfloxacin, and is also active against anaerobes.2 Many treatment resistant Streptococcus
pneumoniae isolates are susceptible to moxifloxacin, although it is not funded for this indication. Moxifloxacin should
not be considered effective against Pseudomonas aeruginosa. While it is not first-line, it does have activity against
susceptible methicillin-resistant Staphylococcus aureus.3
There are few indications for use in primary care
A restrictive approach to the use of quinolones is recommended as community prescribing of quinolones significantly contributes
to antimicrobial resistance (see: “Quinolone resistance is increasing”). Ideally, quinolones should
be reserved for serious, life-threatening or difficult-to-treat infections, when other antibiotics cannot be used due to
allergy or intolerance, or when the pathogen is resistant to alternative antimicrobial agents (see Table
1).
For further information on prescribing quinolones for individual conditions as per Table 1,
including dosing and regimen recommendations, see: https://bpac.org.nz/antibiotics/guide.aspx
Clinical scenarios where ciprofloxacin is not recommended
Ciprofloxacin should not be used for:
- Pneumococcal pneumonia – it does not cover Streptococcus pneumoniae adequately
- Repeat courses for chronic prostatitis if bacterial involvement not confirmed – chronic prostate pain is frequently
not due to infection
- Travellers’ diarrhoea – antibiotic treatment is not typically required as the infection is usually self-limiting and
may be caused by bacteria, viruses or protozoa. Patients with severe or persistent symptoms should be discussed with an
Infectious Diseases Physician or Clinical Microbiologist to decide on an appropriate treatment regimen, depending on the
causative pathogen. Azithromycin is often a recommended choice.
- Diverticulitis - anecdotally, patients are often treated with ciprofloxacin, however, the recommended first-line regimen
is trimethoprim + sulfamethoxazole and metronidazole; amoxicillin clavulanate is an alternative.4
Ciprofloxacin is generally not recommended for pyelonephritis, although it is used occasionally. Trimethoprim + sulfamethoxazole
is first-line; amoxicillin clavulanate and cefalexin are alternatives.4
N.B. Norfloxacin should not be used for pyelonephritis as it has poor tissue penetration and is no longer recommended
for uncomplicated urinary tract infection. Some DHBs have excluded norfloxacin from their formularies as it is no longer
considered appropriate due to resistance and safety concerns.
Table 1. Indications for quinolones in primary care. N.B. There are no indications for norfloxacin in primary care.1, 4–8
|
Ciprofloxacin
|
Moxifloxacin
|
- Epididymo-orchitis – first-line if a UTI pathogen is suspected
- Prostatitis – first-line for acute and chronic bacterial prostatitis
- Otitis externa with secondary infection – only if Pseudomonas is suspected*
- Chronic suppurative otitis media [unapproved indication]†
- Bacterial keratitis or severe bacterial conjunctivitis resistant to chloramphenicol†
- Gonorrhoea – only if isolate is known to be susceptible and an alternative to first-line treatment is required
- Chronic relapsing UTI in adults – fourth-line if treatment with nitrofurantoin, trimethoprim
or cefalexin has failed or the organism is not sensitive‡
- Salmonella enterocolitis - first-line for severe infection, those who are immunocompromised or have prosthetic vascular grafts
- Salmonella typhi and S. paratyphi – if isolate is known to
be susceptible
- Campylobacter enterocolitis – second-line after erythromycin for severe or prolonged
infection, or those at high risk of complications
- Shigellosis – only if severe and isolate is known to be sensitive
- Other indications include invasive Pseudomonas infections, Legionella pneumonia,
bone and joint infections and prophylaxis of meningococcal disease, when no alternative is available
|
- Mycoplasma genitalium urethritis** [unapproved indication] – first-line is doxycycline
(to reduce bacterial load) followed by either azithromycin or moxifloxacin (if macrolide resistant or treatment with azithromycin has failed).
Neither azithromycin nor moxifloxacin are recommended first-line.
|
* Ear drops formulated with hydrocortisone (not funded)
† Eye drops are subsidised by endorsement when prescribed for the treatment of bacterial keratitis or severe bacterial
conjunctivitis resistant to chloramphenicol; or for the second-line treatment of chronic suppurative otitis media (unapproved
indication).
** Moxifloxacin can be prescribed fully funded with Special Authority approval
for the treatment of M. genitalium infection
(unapproved indication). Applications are to be made by a sexual health specialist or on their recommendation. N.B. A similar
regimen is likely to be appropriate for persistent cervicitis or severe pelvic inflammatory disease caused by M. genitalium infection.
‡ Consider the underlying cause of relapsing UTI, e.g. a prostatic abscess or renal tract abnormality
For further information on Mycoplasma genitalium, see:
https://bpac.org.nz/2019/mycoplasma-genitalium.aspx
Quinolones are generally well tolerated, with the most common adverse effects resulting from gastrointestinal disturbance,
as with most antibiotics. Less frequently, people using quinolones may experience central nervous system effects
(e.g. headache, insomnia, dizziness, anxiety, restlessness, tremor), crystalluria*, rash or photosensitivity.18
In rare circumstances, serious adverse effects can occur, including tendinitis and tendon rupture, progression or rupture
of an aortic aneurysm or aortic dissection, QT prolongation, retinal detachment, CNS excitation and seizures (see: “Tendinitis
and tendon ruptures are a rare adverse effect” and “Caution is required when prescribing quinolones in some
patients”).2,19 The
risk of serious adverse effects seems to be greater with later generation quinolones (i.e. moxifloxacin) than with earlier
generations (i.e. ciprofloxacin and norfloxacin).18
* The formation of crystals in the urine due to poor hydration and urine alkalinity; the condition is usually benign,
but there have been reported cases of renal failure associated with crystal precipitation 20, 21
Patients should be advised about the risks so that they can prevent or minimise the impact of any adverse effects if they
occur.
Advise patients to:
- Increase fluid intake to reduce
the risk of crystalluria
- Apply sunscreen or cover exposed areas of skin when outdoors to avoid a photosensitivity reaction
- Stop taking the quinolone and consult with a health professional if tendon pain or swelling occurs, or symptoms of neuropathy,
e.g. pain, burning, tingling, numbness or weakness
- Report any neurological symptoms, e.g. confusion, anxiety, restlessness, to a health professional
N.B. Prescribers should report adverse reactions to the Centre for Adverse Reactions Monitoring (CARM). Reports can be
made through your Adverse Reaction Reporting tool in your patient management system or via a variety of other methods. For
further information, see: https://nzphvc.otago.ac.nz/reporting/
Patient information leaflets are available from the New Zealand Formulary: https://www.nzf.org.nz/nzf_70421
Caution is required when prescribing quinolones in some patients
Many of the adverse effects associated with quinolones occur more frequently in people with pre-existing risk factors,
or in certain at-risk groups, including older people and those with epilepsy.
Older people
Quinolones should be used at the lowest effective dose in older people for as short a duration as clinically possible,
to reduce the development of resistance and adverse effects. Renal function declines consistently with age and ciprofloxacin
and norfloxacin doses need to be reduced accordingly to avoid adverse effects. For example, an appropriate oral dose for
ciprofloxacin in renal impairment is 250–500 mg, twice daily, if eGFR is 30–60 mL/minute/1.73 m2 or once daily,
if eGFR < 30 mL/minute/1.73 m2.1
Many antibiotic classes are associated with adverse CNS effects; these appear to be more common with quinolones than other
systemic antimicrobials and are of particular concern in older people.24 Some adverse CNS effects in older
people may be attributed to ageing, acute illness, other conditions or other medicines so it is important to consider quinolone
use when CNS symptoms are reported.
People with epilepsy or a history of CNS disorders
Quinolones should be used with caution in people at increased risk of seizures, those with CNS disorders or in patients
concurrently using medicines which may lower the seizure threshold, e.g. bupropion, due to the potential for adverse CNS
effects.25 The risk of seizures, although very rare, may be increased with concomitant NSAID treatment.25
People at risk of aortic aneurysm or dissection
A similar mechanism relating to collagen degradation with quinolone treatment that leads to tendon rupture (see: “Tendinitis
and tendon ruptures are a rare adverse effect“) may occur in the wall of the aorta, contributing to an approximately
two-fold increase in the risk of progression or rupture of an aortic aneurysm or aortic dissection within 60 days following
treatment.15,
26 This is a rare effect and as of March 2019, no cases were reported in New Zealand.15
Risk factors include:15, 19, 27
- Family history of aneurysm
- Pre-existing aortic aneurysm or
dissection
- Certain pre-disposing conditions,
e.g. Marfan syndrome, vascular Ehlers-Danlos syndrome, Takayasu's arteritis, giant cell arteritis,
Behçet’s disease
- Atherosclerosis
- Hypertension
- Age > 65 years
Quinolones should only be prescribed to people with these risk factors if there are no suitable alternatives and the benefits
of treatment outweigh the potential harms. Patients should be advised to seek urgent medical advice if they develop sudden-onset,
severe chest, abdominal or lower back pain during or following treatment.19
Tendinitis and tendon ruptures are a rare adverse effect
A number of toxicological studies have confirmed that quinolones damage the collagen within tendons, which on rare occasions can result in tendinitis
and tendon rupture, particularly affecting the Achilles tendon with bilateral involvement possible. This can occur even after a single dose of quinolone and
the risk can persist for months.22 Tendon rupture has been reported within 48 hours of starting treatment, however, cases have also been
reported several months after stopping treatment.22
Risk factors for tendon disorders associated with the use of quinolones include:1, 19, 23
- Age over 60 years
- Concomitant oral corticosteroid treatment
- Chronic kidney disease
- Previous kidney, heart or lung transplant
- Prior history of tendon damage
Although this adverse effect is rare (estimated incidence rate 0.14% to 0.40%),22 it is important to remember that:1
- Quinolones are contraindicated in patients with a history of tendon disorders related to previous quinolone use
- If tendinitis is suspected, the quinolone should be discontinued immediately
Between 2007 and 2012, CARM received 53 reports of tendon disorders associated with quinolone use.14 Over one-third (36%) were reports of tendon ruptures, the
remainder were mainly categorised as tendinitis. The majority (83%) of cases were reported in people aged 60 years and over.
Medicine interactions
Quinolones can interact with a number of other medicines, such as those that reduce seizure threshold, prolong the QT
interval, warfarin and medicines metabolised by common pathways in the liver. For further details on medicines that interact
with quinolones, refer to the NZF Stockley’s interactions checker: https://www.nzf.org.nz
Quinolones should be used cautiously in patients taking warfarin as these medicines may interact to increase the international
normalised ratio (INR) and cause severe bleeding. If a quinolone is the most appropriate treatment option, monitor the INR
three days after initiating antibiotic treatment.1
Other at-risk groups
Caution is also required with quinolone use in people with:1
- Diabetes – glucose levels may be
increased or decreased
- Myasthenia gravis – symptoms may
be exacerbated
- G6PD deficiency – increased risk
of haemolytic anaemia
Quinolones are generally not used in children
Quinolones are not recommended for use in people aged under 18 years as they have been associated with arthropathy and
damage to immature cartilage of weight-bearing joints in animal studies.1 There are some specific circumstances,
such as pseudomonal infections associated with cystic fibrosis, where the short-term use of ciprofloxacin may be justified
in children.28
Quinolones should be avoided in pregnancy and while breastfeeding
All quinolones should be avoided in pregnancy as they have been shown to cause arthropathy in animal studies.1 There
are limited data available on the safety of quinolone use while breastfeeding. The manufacturers recommend avoiding use
as small amounts are detected in the breast milk.1
Quinolone resistance is increasing
Antimicrobial resistance to quinolones is prevalent globally, and includes both Gram-negative
and Gram-positive strains. In New Zealand, resistance has been shown in:
- Haemophilus influenzae – susceptibility testing of 83 isolates by the Institute of Environmental Science and
Research (ESR) in 2017 found 2.4% were resistant to ciprofloxacin9
- Neisseria gonorrhoeae – susceptibility testing of 425 isolates by the ESR in 2015 found 32% were resistant
to ciprofloxacin10
- Mycoplasma genitalium – studies conducted in 2017 and 2020 reported 19–27% of 115 and 81 M. genitalium isolates,
respectively, had mutations associated with increased resistance to quinolones11,12
- Shigella – a 2018 study reported 23% of 263 Shigella isolates were resistant to ciprofloxacin and
norfloxacin13
Ciprofloxacin and norfloxacin use in New Zealand is decreasing
Dispensing data from the last five years show that ciprofloxacin and norfloxacin use has been steadily decreasing (Figures
1 A and B). Increased awareness of the harms of quinolone treatment, as well as education on rational use, may help to explain
this prescribing trend. There have also been changes to funding endorsement for norfloxacin. Medsafe has published two Prescriber
Updates on quinolones since 2012, highlighting the risks of tendon rupture and aortic aneurysm or dissection.14, 15 In
2016, the United States Food and Drug Association revised the warnings for quinolones due to the potential for disabling
and potentially permanent adverse effects.16 In 2018, several news media articles on the use and safety of quinolones
were published in New Zealand.
In most DHBs, ciprofloxacin and norfloxacin dispensing decreased by 25–50% between 2015 and 2019 (Figure 2).17 The
only DHB without a decrease in ciprofloxacin and norfloxacin dispensing was West Coast. In 2019, ciprofloxacin and norfloxacin
use was highest in Midcentral DHB (17 people per 1,000 population) and lowest in Hutt and Counties Manukau DHBs (7 people
per 1,000 population).
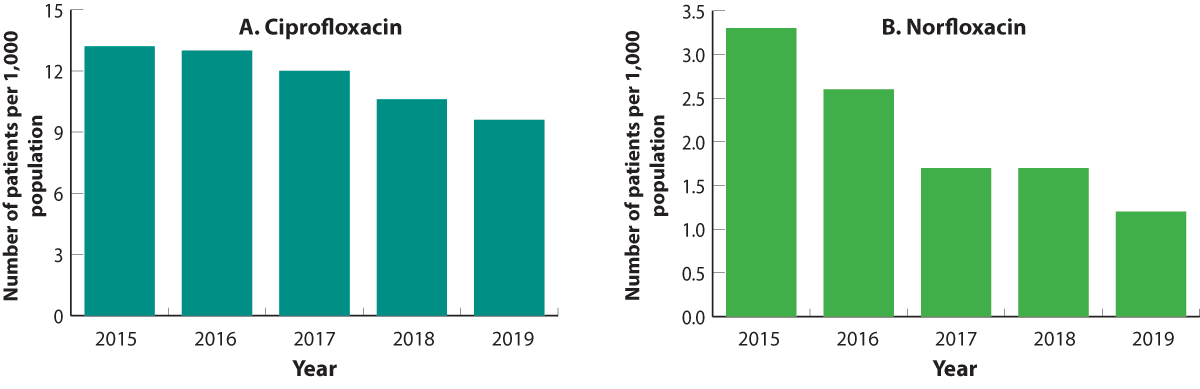
Figure 1 A and B. Number of patients (per 1,000 enrolled patients) dispensed ciprofloxacin (A) or norfloxacin (B), 2015–2019.
Note the different scale on the Y axes.
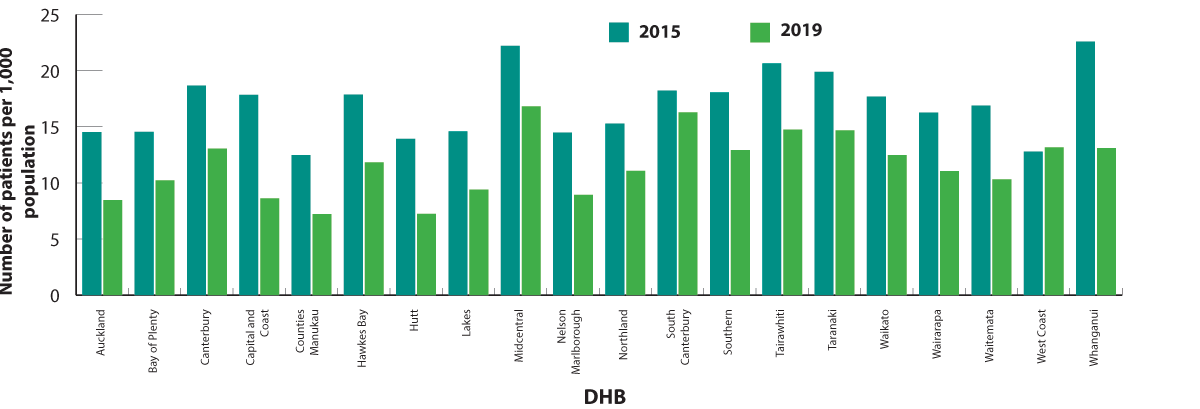
Figure 2. Number of patients (per 1,000 enrolled patients) who were dispensed ciprofloxacin or norfloxacin in 2015 and 2019, by DHB.
Moxifloxacin use is increasing, but total numbers are still small
Moxifloxacin is funded with Special Authority approval for active tuberculosis, Mycoplasma
genitalium infection and penetrating eye injury. The majority of moxifloxacin prescribing for these indications will
occur in secondary care; only applications for M. genitalium can be made by a primary care clinician, but this
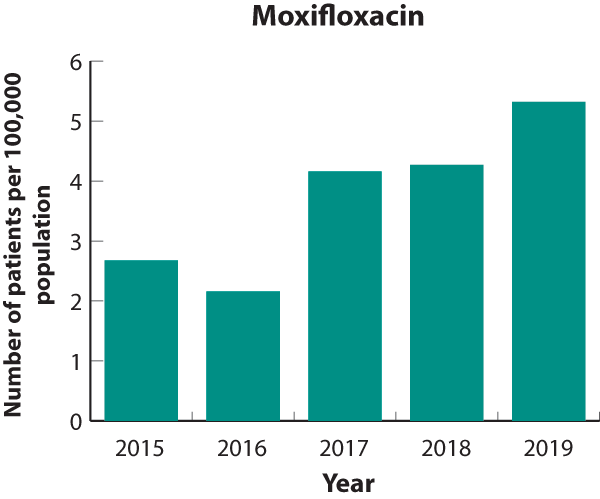
must be on the recommendation of a sexual health physician. Moxifloxacin use more than doubled between 2015 and 2019, however,
the total number of patients dispensed moxifloxacin is still very low (i.e. 251 people in total were dispensed moxifloxacin
in 2019) (Figure 3). Moxifloxacin dispensing increased in most DHBs between 2015 and 2019 (Figure 4). The highest dispensing
rate in 2019 was in Taranaki DHB (17 people per 100,000 population). Possible reasons for this increase include:
- Treatment of infections caused by multi-resistant S. pneumoniae
- Increased awareness and laboratory detection of M. genitalium infection as a cause of urethritis, cervicitis
and pelvic inflammatory disease
- An outbreak of tuberculosis where first-line treatments were inappropriate due to resistance or intolerance
Figure 3. Patients dispensed moxifloxacin
(per 100,000 enrolled patients), 2015–2019. Note the scale on the
Y axis has been adjusted to represent the comparatively small population of patients dispensed moxifloxacin. |
|
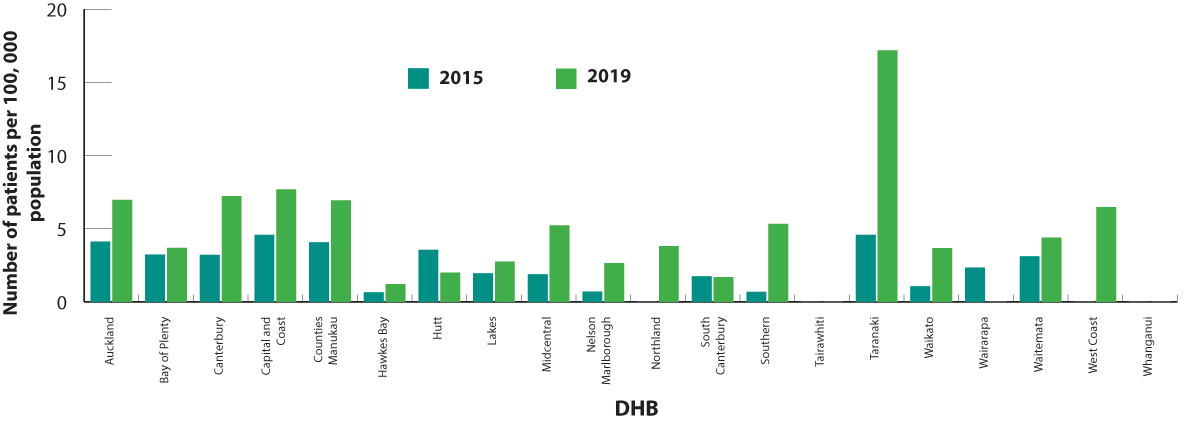
Figure 4. Number of patients (per 100,000 enrolled patients) who were dispensed moxifloxacin in 2015 and 2019, by DHB.
Peer group discussion available
A peer group discussion is available that is related to this article. See peer group discussion
Peer group discussion sheets are available on our website and aim to provide discussion points for use within your
peer group. To claim CPD credits for peer review activities, the RNZCGP requires peer groups to be registered. As with
other CPD activities one hour of learning activity equates to one credit.





