This audit helps healthcare professionals identify patients with type 2 diabetes who are at high risk of cardiovascular or renal complications and meet the Special Authority criteria for funded treatment with either a SGLT-2 inhibitor (i.e. empagliflozin) or a GLP-1 receptor agonist (dulaglutide or liraglutide), to ensure that their diabetes management regimen is optimised to include one of these medicines.
As of December, 2024, the Special Authority criteria for funded treatment with empagliflozin have been widened to include patients with symptomatic heart failure with reduced ejection fraction (HFrEF), regardless of their diabetes status. This audit, however, focuses on the treatment of patients with type 2 diabetes.
For further information on the use of empagliflozin for people with heart failure, see: https://bpac.org.nz/2025/heart-failure-part-2.aspx
Sodium-glucose co-transporter 2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists are widely recommended as part of the management of patients with type 2 diabetes and have been funded in New Zealand since 2021. The SGLT-2 inhibitor empagliflozin is funded for patients with type 2 diabetes* who meet Special Authority criteria. Initially, only one GLP-1 receptor agonist, dulaglutide, was funded for patients with type 2 diabetes who met Special Authority criteria. However, due to ongoing global supply issues, funding was extended to include a second GLP-1 receptor agonist, liraglutide.
The “no new patient” restrictions on GLP-1 receptor agonists implemented in May, 2024, have been removed from liraglutide (March, 2025) and dulaglutide (July, 2025). Therefore, new patients with type 2 diabetes who meet the Special Authority criteria for funded treatment with a GLP-1 receptor agonist can be initiated on either liraglutide (Victoza†) or dulaglutide (Trulicity).
* Empagliflozin is also funded for patients with symptomatic HFrEF (regardless of diabetes status); separate Special Authority criteria apply
† Victoza is the only brand of liraglutide funded with Special Authority approval for patients with type 2 diabetes. Other brands of liraglutide, e.g. Saxenda, are approved for use in New Zealand as adjunctive treatments for weight loss (not funded).
Special Authority criteria for funded empagliflozin or GLP-1 receptor agonist treatment
The Special Authority criteria for funded treatment with empagliflozin or a GLP-1 receptor agonist (dulaglutide or liraglutide) for type 2 diabetes were originally the same but now differ depending on the blood-glucose lowering medicines the patient has previously trialled (Table 1). To meet the Special Authority criteria for a GLP-1 receptor agonist, a patient must first have trialled treatment with empagliflozin, where clinically appropriate.
Table 1. Special Authority criteria for funded treatment with empagliflozin or a GLP-1 receptor agonist (dulaglutide or liraglutide) for type 2 diabetes.
Patient has type 2 diabetes and at least one of the following characteristics:
- Māori or any Pacific ethnicity; or
- Pre-existing cardiovascular disease (CVD) or risk equivalent*; or
- An absolute five-year CVD risk of ≥ 15% according to a validated cardiovascular risk assessment calculator; or
- A high lifetime cardiovascular risk due to being diagnosed with type 2 diabetes during childhood or as a young adult; or
- Diabetic kidney disease†
* Defined as: prior cardiovascular disease event (i.e. angina, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, transient ischaemic attack, ischaemic stroke, peripheral vascular disease), congestive heart failure or familial hypercholesterolaemia
† Defined as: persistent albuminuria (albumin:creatinine ratio ≥ 3 mg/mmol, in at least two out of three samples over a 3 - 6 month period) and/or eGFR < 60 mL/min/1.73 m2 in the presence of diabetes, without alternative cause |
AND
HbA1c level remains > 53 mmol/mol |
To qualify for funded
empagliflozin |
To qualify for a funded
GLP-1 receptor agonist (dulaglutide or liraglutide)
|
- Have trialled at least one blood-glucose lowering medicine (e.g. metformin, vildagliptin or insulin) for at least three months
OR
- Have previously received approval for a GLP-1 receptor agonist
|
Have trialled all of the following blood-glucose lowering medicines for at least six months, where clinically appropriate: empagliflozin, metformin and vildagliptin |
N.B. Funded treatment with both empagliflozin and a GLP-1 receptor agonist is not available, unless the patient is receiving empagliflozin (alone or in combination with metformin) for the treatment of HFrEF.
Patients who are likely to benefit from treatment, but do not meet the relevant Special Authority criteria
While the Special Authority criteria for empagliflozin and GLP-1 receptor agonists (dulaglutide and liraglutide) for type 2 diabetes ensure access for patients who are at high risk of cardiovascular and renal disease, there are some patients who are likely to benefit from treatment who do not meet these criteria, including those:
- With CVD (or five-year CVD risk ≥ 15%), renal disease or heart failure* with a HbA1c < 53 mmol/mol or eGFR 60 – 90 mL/min/1.73 m2 without albuminuria
- With CVD (or five-year CVD risk ≥ 15%) or renal disease who are already taking funded empagliflozin or a GLP-1 receptor agonist (i.e. dual treatment with these medicines is recommended if HbA1c levels remain above target or the patient is likely to derive additional benefit, but only one can be funded at a time, except in patients with heart failure)
- With obesity who have a HbA1c above target despite regular use of metformin (or inability to tolerate it), but who do not have cardiovascular or renal disease† and are not of Māori or Pacific ethnicity
- With a HbA1c above target despite regular use of metformin and vildagliptin (or inability to tolerate them), but who do not have cardiovascular or renal disease† and are not of Māori or Pacific ethnicity
- With a HbA1c within the target range but where a SGLT-2 inhibitor is preferred to avoid adverse effects associated with other options, e.g. hypoglycaemia with a sulfonylurea, weight gain with pioglitazone
Discuss the recommendations with patients and the option to self-fund treatment, unless there are contraindications or significant cautions. This may be a challenging conversation to negotiate as there will be patients who are unable to meet the financial burden of self-funding treatment and may find this distressing. However, it is important that patients are aware of their options in order to make an informed decision about their health care.
* Only applies to GLP-1 receptor agonists; as of December, 2024, the empagliflozin Special Authority criteria have been widened to include patients with symptomatic HFrEF regardless of their diabetes status
† Some patients may have cardiovascular and/or renal risk factors but not meet the clinical thresholds outlined in the Special Authority criteria
Choosing between empagliflozin and a GLP-1 receptor agonist
The choice between funded treatment with empagliflozin or a GLP-1 receptor agonist is determined by the Special Authority criteria. Treatment with empagliflozin must be trialled first; if the patient’s HbA1c remains > 53 mmol/mol despite at least six months of treatment, the patient will then meet the Special Authority criteria for a GLP-1 receptor agonist (dulaglutide or liraglutide).
For patients who meet the Special Authority criteria for both or who are self-funding treatment, there are some clinical scenarios where one medicine may be superior to the other. In patients with heart failure or diabetic kidney disease, a SGLT-2 inhibitor is preferred, while a GLP-1 receptor agonist is likely to induce greater weight loss and improvements in glycaemic control and therefore may be more valuable for patients with obesity. Patient preference is another consideration, particularly regarding the route of administration, i.e. oral versus once weekly (dulaglutide) or daily (liraglutide) injection.
Dual treatment with empagliflozin and a GLP-1 receptor agonist (dulaglutide or liraglutide) is ideally the next step for patients who continue to have a HbA1c level above target while taking one of these medicines alone. However, this is not funded unless the patient is receiving empagliflozin (alone or in combination with metformin) for the treatment of heart failure.
For further information on empagliflozin and GLP-1 receptor agonists in type 2 diabetes, see: https://bpac.org.nz/2021/diabetes.aspx
Summary
This audit identifies patients with type 2 diabetes who are at high risk of cardiovascular or renal complications and meet the relevant Special Authority criteria for funded treatment with either empagliflozin or a GLP-1 receptor agonist (dulaglutide or liraglutide) to ensure that their diabetes management regimen is optimised to include one of these medicines.
N.B. This audit can be modified to exclude patients already prescribed either empagliflozin or a GLP-1 receptor agonist so only those who may benefit from adding these medicines are identified; subsequent audit steps will require modification as appropriate.
Recommended audit standards
Ideally, all adult patients with type 2 diabetes who are at high risk of cardiovascular or renal complications and meet the relevant Special Authority criteria will be prescribed either empagliflozin or a GLP-1 receptor agonist as part of their diabetes management regimen.
As these medicines have been funded (with Special Authority approval) for patients with type 2 diabetes since 2021, it is expected that most patients who are at high risk of complications will already be prescribed one of the funded treatment options, unless there is a clinical reason why treatment is not appropriate. However, if this is not the case a follow-up audit (second cycle) should subsequently be completed; the number of patients appropriately prescribed funded treatment should be higher, as any who met the relevant Special Authority criteria but were not receiving funded treatment in the first cycle may have since been prescribed it. N.B. May not be applicable if the audit is modified to exclude patients already prescribed either empagliflozin or a GLP-1 receptor agonist.
Additional audit cycles are also valuable for identifying patients who:
- Did not meet the relevant Special Authority criteria for either empagliflozin or a GLP-1 receptor agonist at the time of the first cycle of the audit, but do at the time of the next cycle and can therefore be prescribed funded treatment
- Only met the Special Authority criteria for empagliflozin at the time of the first cycle, but at the time of the next cycle subsequently meet the Special Authority criteria for funded treatment with a GLP-1 receptor agonist
- Have been diagnosed with type 2 diabetes since completion of the first cycle of the audit and meet the relevant Special Authority criteria for funded treatment with either empagliflozin or a GLP-1 receptor agonist
 Alternatively, consider a "working audit" where the data sheet is completed over time when any eligible patient presents. Document whether they meet the relevant Special Authority criteria for either empagliflozin or a GLP-1 receptor agonist, and if so, whether treatment has already been initiated. Patients who are not currently prescribed funded treatment despite meeting Special Authority criteria should be flagged for review (with you or another clinician), unless there is a clinical reason why treatment is not appropriate.
Alternatively, consider a "working audit" where the data sheet is completed over time when any eligible patient presents. Document whether they meet the relevant Special Authority criteria for either empagliflozin or a GLP-1 receptor agonist, and if so, whether treatment has already been initiated. Patients who are not currently prescribed funded treatment despite meeting Special Authority criteria should be flagged for review (with you or another clinician), unless there is a clinical reason why treatment is not appropriate.
Eligible patients
Any patient aged over 18 years with type 2 diabetes and a HbA1c > 53 mmol/mol is eligible for this audit.
Identifying patients
You will need to have a system in place that allows you to identify eligible patients and audit their clinical notes. Many practices will be able to do this by running a “query” through their PMS to find patients aged over 18 years with a diagnosis of type 2 diabetes and a HbA1c > 53 mmol/mol.
If conducting a working audit, fill in the data sheet when you have a consultation for any reason with an eligible patient over the course of an allocated time frame.
Sample size
The number of eligible patients will vary according to your practice demographic. If a large number of results are returned, a sample size of 30 patients is sufficient for this audit. However, all eligible patients should be reviewed subsequently over time.
If conducting a working audit, a smaller sample size may be necessary to complete it within your planned time frame.
Criteria for a positive outcome
A positive result is any patient who meets the relevant Special Authority criteria for funded treatment with either empagliflozin or a GLP-1 receptor agonist (dulaglutide or liraglutide; Table 1) and is:
- Currently prescribed the medicine, OR
- Not currently prescribed the medicine due to clinical reasons, e.g. intolerance, treatment has been trialled previously and was unsuccessful
Any patients who are not currently prescribed either empagliflozin or a GLP-1 receptor agonist despite meeting Special Authority criteria should be flagged for review, unless there is a clinical reason why treatment is not appropriate.
Although they are not the focus of this audit, patients who are likely to benefit from treatment with empagliflozin or a GLP-1 receptor agonist but do not meet the Special Authority criteria for funded treatment may also be identified. Consider flagging these patients for a discussion about treatment options, including the possibility of self-funding.
If conducting a working audit, and the patient is not currently prescribed either empagliflozin or a GLP-1 receptor agonist despite meeting the relevant Special Authority criteria, a review should be undertaken at the time, or a future appointment booked to review their treatment, unless there is a clinical reason why treatment is not appropriate.
Data analysis
Use the sheet provided to record your data. The percentage achievement can be calculated by dividing the number of patients with a positive result (i.e. sum of ‘YES‘ in column C + ‘YES‘ in column D) by the total number of patients who are eligible for funded treatment (i.e. sum of ‘YES‘ in column B).
N.B. Consider flagging patients who do not meet the Special Authority criteria for either empagliflozin or a GLP-1 receptor agonist, i.e. those with a ‘NO‘ in column B, for a discussion about the possibility of self-funding treatment.
Clinical audits can be an important tool to identify where gaps exist between expected and actual performance. Once completed, they can provide ideas on how to change practice and improve patient outcomes. General practitioners are encouraged to discuss the suitability and relevance of their proposed audit with their practice or peer group prior to commencement to ensure the relevance of the audit. Outcomes of the audit should also be discussed with the practice or peer group; this may be recorded as a learning activity reflection if suitable.
The Plan, Do, Study, Act (PDSA) model is recommended by the Royal New Zealand College of General Practitioners (RNZCGP) as a framework for assessing whether a clinical audit is relevant to your practice. This model has been widely used in healthcare settings since 2000. It consists of two parts, the framework and the PDSA cycle itself, as shown in Figure 1.
1. The framework
This consists of three questions that help define the “what” and “how” of an improvement project (in this case an audit).
The questions are:
- "What are we trying to accomplish?" – the aim
- "How will we know that a change is an improvement?" – what measures of success will be used?
- "What changes can we make that will result in improvement?" – the concept to be tested
2. The PDSA cycle
This is often referred to as the “engine” for creating, testing and carrying out the proposed changes. More than one cycle is usually required; each one is intended to be short, rapid and frequent, with the results used to inform and refine the next. This allows an ongoing process of continuous learning and improvement.
Each PDSA cycle includes four stages:
- Plan – decide what the change to be tested is and how this will be done
- Do – carry out the plan and collect the data
- Study – analyse the data, assess the impact of the change and reflect on what was learned
- Act – plan the next cycle or implement the changes from your plan
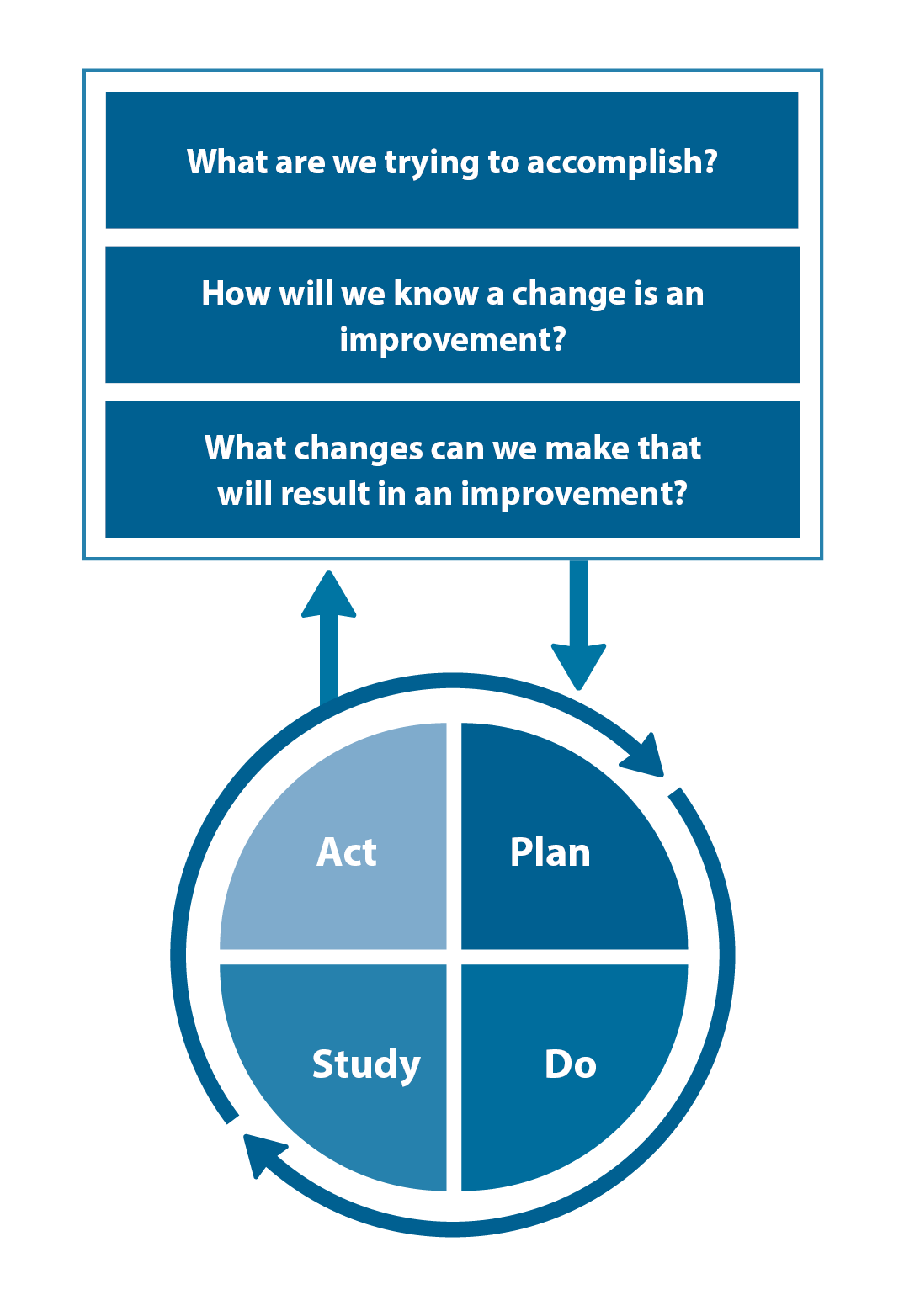
Figure 1. The PDSA model for improvement.
Source: Plan, Do, Study, Act (PDSA) cycles and the model for improvement

Claiming credits for Te Whanake CPD programme requirements
Practice or clinical audits are useful tools for improving clinical practice and credits can be claimed towards the Patient Outcomes (Improving Patient Care and Health Outcomes) learning category of the Te Whanake CPD programme, on a two credit per learning hour basis. A minimum of 12 credits is required in the Patient Outcomes category over a triennium (three years).
Any data driven activity that assesses the outcomes and quality of general practice work can be used to gain credits in the Patient Outcomes learning category. Under the refreshed Te Whanake CPD programme, audits are not compulsory and the RNZCGP also no longer requires that clinical audits are approved prior to use. The college recommends the PDSA format for developing and checking the relevance of a clinical audit.
To claim points go to the RNZCGP website: www.rnzcgp.org.nz
If a clinical audit is completed as part of Te Whanake requirements, the RNZCGP continues to encourage that evidence of participation in the audit be attached to your recorded activity. Evidence can include:
- A summary of the data collected
- An Audit of Medical Practice (CQI) Activity summary sheet (Appendix 1 in this audit or available on the
RNZCGP website).
N.B. Audits can also be completed by other health professionals working in primary care (particularly prescribers), if relevant. Check with your accrediting authority as to documentation requirements.
