Published: December, 2024 | Review date: December, 2027
This audit helps health professionals in primary care identify patients who are prescribed menopausal hormone therapy (MHT) to assess whether they have had a review of the benefits and risks when initiating or continuing treatment in the past 12 months.
MHT is the most effective treatment for the vasomotor symptoms and urogenital atrophy associated with menopause, however, it is associated with an increased risk of adverse outcomes such as breast cancer, stroke and venous thromboembolism (VTE). The extent of risk depends on the timing or age at initiation, MHT type, dose, duration of use,* route of administration and whether a progestogen is used (and which one).
* Stroke and VTE risk do not appear to be influenced by the duration of treatment, but are influenced by age
The benefit-risk ratio of MHT is most favourable for females aged < 60 years or who are within ten years of menopause. The absolute risk of adverse events such as VTE or stroke is low in this group and are likely outweighed by the benefits of treatment for those who have menopause symptoms affecting their quality of life. Non-hormonal treatments, e.g. SSRIs or SNRIs, may be suitable for females with vasomotor symptoms who have a contraindication to MHT or who do not wish to use it. Topical vaginal products are recommended instead of MHT for females who primarily have urogenital symptoms, e.g. moisturisers, lubricants, vaginal oestrogen cream or pessary.
All patients taking MHT should have an annual review to determine whether ongoing treatment is appropriate, which takes into consideration any change in risk factors, e.g. for cardiovascular disease or breast cancer, and the extent of benefit. Guidelines do not recommend an upper limit on the duration of MHT; patients who were initiated on MHT at a younger age can continue treatment beyond age 60 years if they feel it is beneficial and there are no new contraindications, e.g. an oestrogen-dependent cancer or cardiovascular event. Document the symptoms that MHT is being prescribed for in the patient’s notes; assessment of benefit will usually be subjective improvement in these symptoms.
For further information on prescribing MHT and the risks and benefits associated with treatment, see:
Menopausal hormone therapy: where are we now?
Summary
This audit identifies patients prescribed MHT to assess whether they have had a review of the benefits and risks when initiating or continuing treatment in the last 12 months.
Recommended audit standards
Ideally, all patients who are prescribed MHT should have a documented review of the benefits and risks when initiating or continuing treatment in their clinical notes in the past 12 months. If there is no evidence of this, the patient should be flagged for review.
Alternatively, consider a “working audit” where the data sheet is filled in over time opportunistically during consultations for any reason with an eligible patient until the required number of patients has been reached. If conducting a “working audit” and there is no record of an assessment of the benefits and risks of MHT in the last 12 months, this should be undertaken at the time, or a future appointment booked.
Eligible patients
All patients within the practice who have been prescribed MHT in the last 12 months are eligible for this audit.
Identifying patients
You will need to have a system in place that allows you to identify eligible patients and audit their clinical notes. Many practices will be able to do this by running a “query” through their PMS to find all patients prescribed MHT in the last 12 months.
If conducting a “working audit”, fill in the data sheet when you have a consultation for any reason with an eligible patient until the required number of patients has been reached.
Sample size
The number of eligible patients will vary according to your practice demographic. A sample size of 20 to 30 patients is sufficient for this audit. It is unlikely that a large number of results will be returned, but if so, take a random sample of 20 – 30 patients. A smaller sample size may be necessary if conducting a working audit.
Criteria for a positive outcome
For a positive result, the patient’s clinical notes should contain a record of a review of the benefits and risks of MHT when initiating or continuing treatment in the last 12 months.
The benefits of MHT treatment are based on a subjective improvement in symptoms, as reported by the patient. Ongoing benefit after longer term use may only be able to be assessed if MHT treatment has been temporarily stopped and symptoms recurred. Some patients may also have experienced symptoms when changing brands or formulations of MHT, confirming the benefit of their original product. The risk assessment should include:
- The patient’s age
- An update of cardiovascular disease history and risk, e.g. BMI, smoking status, blood pressure, diabetes, any cardiovascular events
- An update of breast cancer history and risk, including whether there is a record of regular mammograms*
- Whether there is any unexplained vaginal bleeding
* Females aged 45 – 69 years are eligible for free mammogram screening every two years
Data analysis
Use the sheets provided to record your data.
In the conventional audit (Data sheet A), a positive result is any patient taking MHT who has evidence in their clinical notes of a review of the benefits and risks when initiating or continuing treatment in the last 12 months. The percentage achievement can be calculated by dividing the number of patients with a positive result by the total number of patients audited. If there is no documented assessment, the patient should be flagged for review.
In a working audit (Data sheet B), aim to carry out an assessment of the benefits and risks of MHT for as many eligible patients as possible.
Clinical audits can be an important tool to identify where gaps exist between expected and actual performance. Once completed, they can provide ideas on how to change practice and improve patient outcomes. General practitioners are encouraged to discuss the suitability and relevance of their proposed audit with their practice or peer group prior to commencement to ensure the relevance of the audit. Outcomes of the audit should also be discussed with the practice or peer group; this may be recorded as a learning activity reflection if suitable.
The Plan, Do, Study, Act (PDSA) model is recommended by the Royal New Zealand College of General Practitioners (RNZCGP) as a framework for assessing whether a clinical audit is relevant to your practice. This model has been widely used in healthcare settings since 2000. It consists of two parts, the framework and the PDSA cycle itself, as shown in Figure 1.
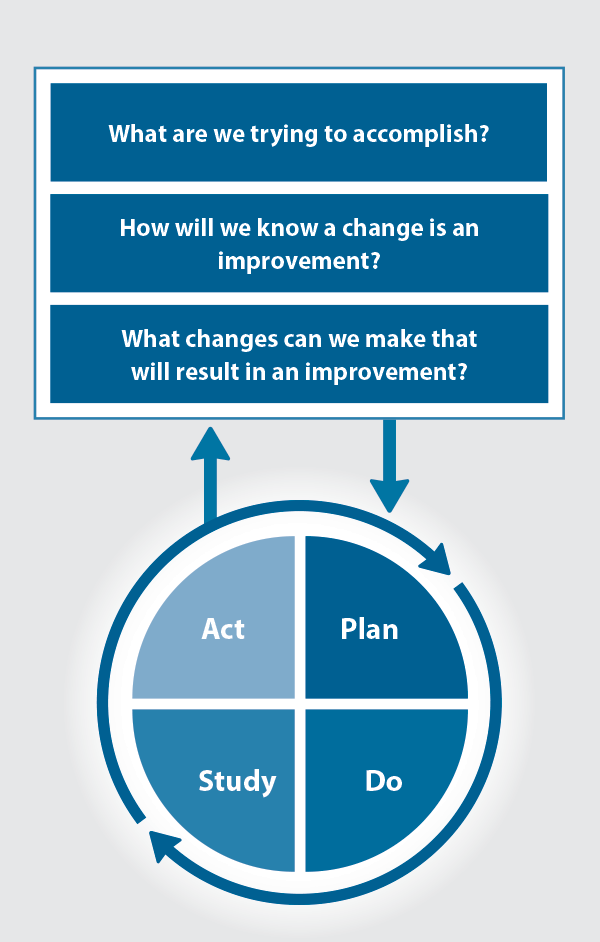
Figure 1. The PDSA model for improvement.
Source: Plan, Do, Study, Act (PDSA) cycles and the model for improvement
1. The framework
This consists of three questions that help define the “what” and “how” of an improvement project (in this case an audit).
The questions are:
- "What are we trying to accomplish?" – the aim
- "How will we know that a change is an improvement?" – what measures of success will be used?
- "What changes can we make that will result in improvement?" – the concept to be tested
2. The PDSA cycle
This is often referred to as the “engine” for creating, testing and carrying out the proposed changes. More than one cycle is usually required; each one is intended to be short, rapid and frequent, with the results used to inform and refine the next. This allows an ongoing process of continuous learning and improvement.
Each PDSA cycle includes four stages:
- Plan – decide what the change to be tested is and how this will be done
- Do – carry out the plan and collect the data
- Study – analyse the data, assess the impact of the change and reflect on what was learned
- Act – plan the next cycle or implement the changes from your plan

Claiming credits for Te Whanake CPD programme requirements
Practice or clinical audits are useful tools for improving clinical practice and credits can be claimed towards the Patient Outcomes (Improving Patient Care and Health Outcomes) learning category of the Te Whanake CPD programme, on a two credit per learning hour basis. A minimum of 12 credits is required in the Patient Outcomes category over a triennium (three years).
Any data driven activity that assesses the outcomes and quality of general practice work can be used to gain credits in the Patient Outcomes learning category. Under the refreshed Te Whanake CPD programme, audits are not compulsory and the RNZCGP also no longer requires that clinical audits are approved prior to use. The college recommends the PDSA format for developing and checking the relevance of a clinical audit.
To claim points go to the RNZCGP website: www.rnzcgp.org.nz
If a clinical audit is completed as part of Te Whanake requirements, the RNZCGP continues to encourage that evidence of participation in the audit be attached to your recorded activity. Evidence can include:
- A summary of the data collected
- An Audit of Medical Practice (CQI) Activity summary sheet (Appendix 1 in this audit or available on the
RNZCGP website).
N.B. Audits can also be completed by other health professionals working in primary care (particularly prescribers), if relevant. Check with your accrediting authority as to documentation requirements.





