Following the introduction of the combined measles, mumps and rubella (MMR) vaccine in 1990, the number of cases of all
three diseases has significantly and progressively declined.1 Since then, there have been very few large outbreaks
and in 2017, the World Health Organization (WHO) verified New Zealand as free of both endemic measles and rubella (see:
“Epidemiology definitions”).1 Historical issues in vaccination coverage, however,
have left many adolescents and young adults in New Zealand more likely to have missed full MMR vaccination and at greatest
risk of infection (see: “Adolescents and young adults in New Zealand are under-immunised”).1,
2 For this reason, the Ministry of Health now recommends that this population group is prioritised for recall for
catch-up MMR vaccination to help to close the immunity gap, reducing the risk of future outbreaks.1
N.B. While adolescents and young adults are currently the priority for catch-up MMR vaccination, anyone born on, or after
1 January, 1969, without documented evidence of immunity to all three diseases may also receive funded catch-up MMR vaccination
(see: “Encourage immunisation of susceptible individuals”).1
Criteria for determining immunity
Evidence of immunity against measles, mumps and rubella requires documented history of two doses of MMR, or serologic
evidence of immunity against all three diseases.1 Clinical history of disease alone does not reliably indicate
immunity.1 Serologic evidence of protection against one disease (if tested), or documented history of measles-only
vaccination, cannot be used as a proxy for immunity against the other two diseases; complete MMR vaccination is still required.1
N.B. In New Zealand, serological testing for immunity against rubella is typically only performed as part
of the “antenatal screen” and serological testing is not funded or recommended for routine use;1 if there is
doubt about vaccination status, it is safe and effective to offer further vaccination rather than a serological test.
Epidemiology definitions
Cluster: a greater than expected accumulation of cases of a health condition (e.g. disease or injury)
which are grouped together in place and time.3 N.B. The expected number of cases is not always known.3
Endemic: the constant presence of a disease, infectious organism or health condition within a given population
and in a given geographic area.3
Epidemic: an increase in the number of cases of a disease, illness, health-related event or health-related
behaviour that far exceeds the expected number within a community, region, or any other group of people at a particular
period.3, 4
Outbreak: technically synonymous to epidemic, outbreak, however, is typically used to describe a more
localised increase in the number of cases of a health condition (e.g. disease or injury), such as within a community, town
or institution.3, 4
Pandemic: an epidemic on a much larger scale, spreading to many countries or continents and therefore
usually affecting a larger number of people around the world.3
Adolescents and young adults in New Zealand are under-immunised
Since the early 1990s, several gaps in MMR coverage have resulted in certain cohorts in New Zealand being under-immunised,
particularly adolescents and young adults aged between 15 and 30 years.1 This gap in immunity is due to a combination
of historical issues in vaccination coverage directly affecting this population group, including:1
-
Historically low national immunisation rates, with less than 60% of children being fully vaccinated by
age two years in the 1991/92 National Childhood Immunisation Survey.5, 6 Coverage increased to 63.1% in 1996
(with lower rates for those of Māori [44.6%] and Pacific [53.1%] ethnicity) and further increased to 77.4% in 2005.5,
6 N.B. National immunisation coverage dropped again in the 2010s but increased to 88% (for children aged five years)
between 2020 – 2021.7
-
Unfounded, but widespread vaccine safety concerns regarding a since discredited association between MMR
vaccine and the development of autism in children in the late 1990s and early 2000s* (see: “Vaccination hesitancy”
for further information on immunisation concerns)8, 9
-
Changes to the timing of the second dose in the MMR vaccination schedule in 2001; from age 11 years to
age four years
-
Potentially compromised vaccine quality, resulting from inadequate cold chain processes, which have now
been rectified
-
Vaccine supply shortages, particularly during the 2019 measles outbreak10, 11
*These claims have been discredited, with over 30 years of epidemiological research confirming
there is no evidence of a link between MMR and autism.8, 9 The original study (1998) was retracted in 2004 due
to dishonest and irresponsible methods, where it was later disclosed to have used incorrect laboratory reports and falsified
patient data. For further information, see:
www.thelancet.com/journals/lancet/article/PIIS0140-6736(10)60175-4/fulltext.1
Recent mumps and measles outbreaks highlight immunity gap
The most recent mumps and measles epidemics in New Zealand occurred in 2016/2017 and 2018/2019, with most cases located
in the wider Auckland region (see: “Measles, mumps and rubella: an overview”).1, 12 Adolescents
and young adults aged between 12 – 29 years* were the population group most affected by the outbreaks (Figure
1).1, 12
During the 2018/2019 measles epidemic, nine outbreaks occurred throughout New Zealand, resulting in 2,213
notified cases, of which:1, 13
-
Six outbreak clusters were linked to imported cases; deriving from
Australia, Thailand, Japan, Singapore and the Philippines
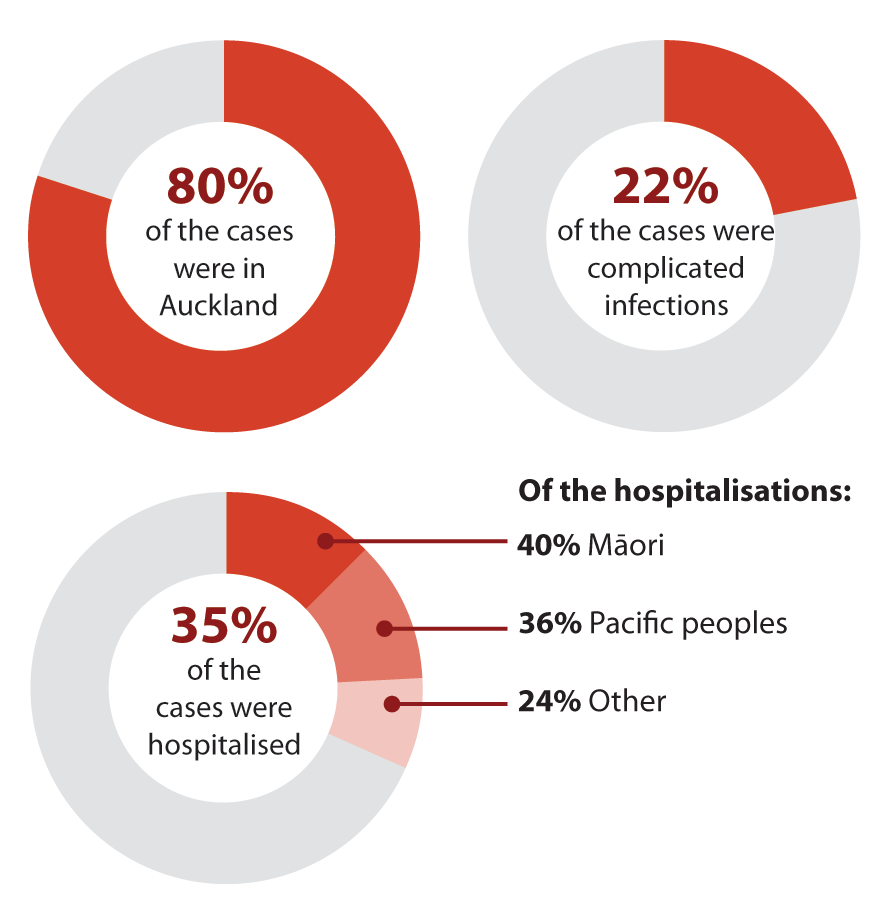
The highest burden of disease was among under-immunised young children, adolescents and young adults;
particularly infants aged under two years and adolescents/young adults aged between ten and 30 years* (see: “Adolescents
and young adults in New Zealand are under-immunised”)
*The age range has been inconsistently reported, i.e. 10 – 15 and 20 – 30 years, and varies depending
on data age groupings1, 13, 14
Rubella infection is still rare in New Zealand
The most recent rubella outbreak occurred between 1995 – 1996, mostly involving young adult males, and likely due to the
earlier under-immunisation of young males (see: “Vaccination history of measles, mumps and rubella in
New Zealand”).1 Since 1998, no incidences of congenital rubella syndrome (CRS), and very few cases of rubella,
have been reported.1 In 2017, New Zealand was verified by the WHO as rubella-free, and since then only three
imported cases of rubella have been reported (Figure 1).1
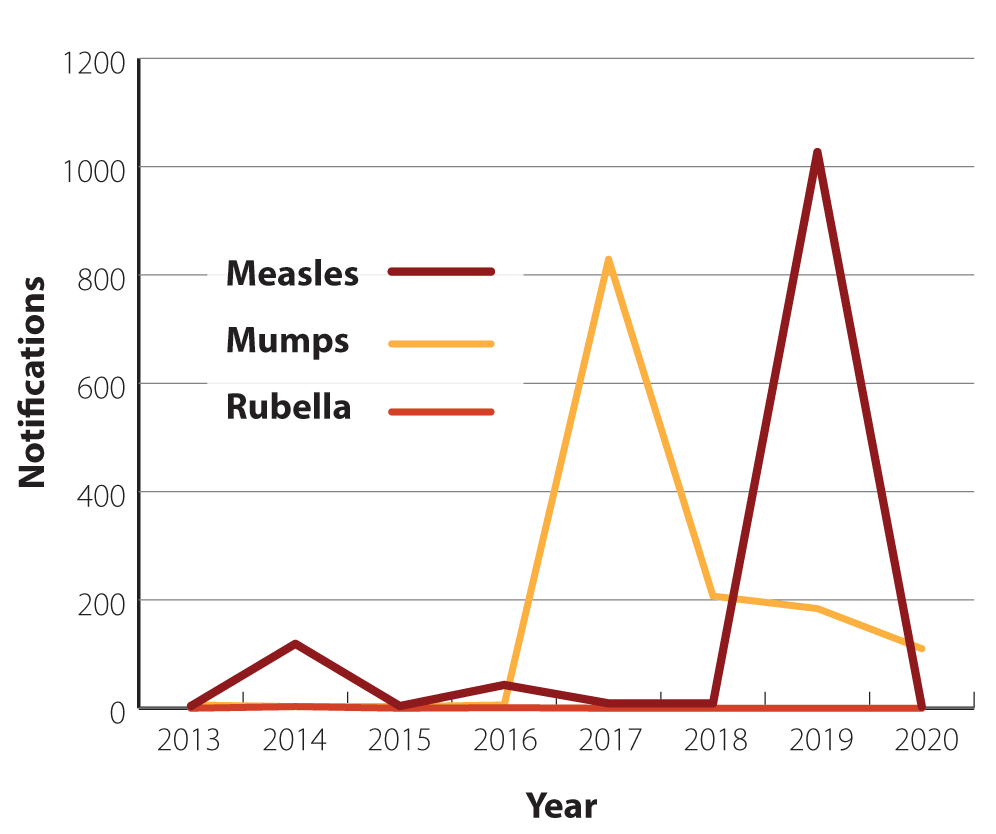
Figure 1. Measles, mumps and rubella notifications for adolescents and young adults aged
15 – 29 years in New Zealand from 2013 – 2020.12
Measles, mumps and rubella: an overview
Measles
Measles is a highly infectious viral disease caused by a paramyxovirus.1 It can be transmitted through both
airborne spread (coughing, sneezing, breathing) and direct person-to-person contact (via transfer of infectious droplets).1,
15, 16 Measles is characterised by clinical features of fever and a distinctive maculopapular rash, and causes an
acute immune suppression that leads to widespread infection.1, 15 After exposure, the virus has an incubation
period of approximately ten days before symptoms appear, typically in three characteristic stages.1, 16
Measles infection increases the risk of further complications such as pneumonia, encephalitis and myocarditis and in rare
cases, sub-acute sclerosing panencephalitis*.1
*Sub-acute sclerosing panencephalitis is a rare but serious and fatal degenerative nervous system
disease, that arises from persistent, wild-type measles infection and typically appears 7 – 11 years following initial infection17
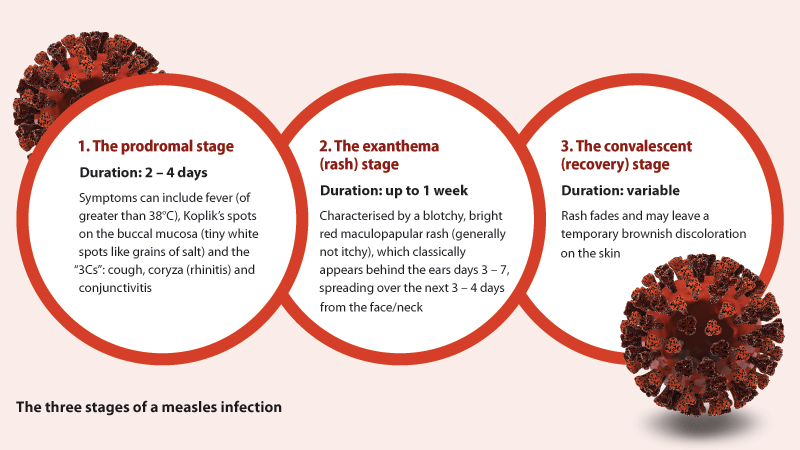
Mumps
Mumps is an acute viral infection also caused by a paramyxovirus, and characterised by clinical features of headache,
fever and parotitis (parotid salivary gland swelling and tenderness).1 It is transmitted both indirectly (through
airborne droplets) and directly (through contact with urine or saliva), and is most infectious for the period of two days
before and five days after onset of parotitis.1 Widespread mumps infection can lead to further complications
such as meningitis, encephalitis, hearing loss, mastitis and oophoritis in females and orchitis in males that can decrease
fertility or lead to temporary sterility; it is unknown whether mumps can result in permanent sterility.1, 18
N.B. Parotid gland swelling is most common, but swelling can also occur in other salivary glands and other structures,
e.g. the brain and testes.1
Rubella
Rubella is an infectious disease caused by a togavirus that affects both adults and children.1 The virus is
comparatively less infectious than measles (Table 1), however, maternal infection can lead to serious
consequences for the unborn child, particularly if contracted during the first trimester of pregnancy.1
Congenital rubella syndrome (CRS) is the most severe complication following rubella and results from
maternal infection during pregnancy.1, 19 CRS is characterised by a number of serious consequences including
miscarriage, fetal death and severe congenital defects including hearing impairment, congenital heart disease, cataracts
and developmental delay.1, 19 Fetal damage can occur in up to 80% of infants if rubella is contracted within
the first 12 weeks of pregnancy, decreasing to 50% after 16 weeks and 25% after the end of the second trimester; multiple
defects are common.1, 19
In cases of highly infectious vaccine-preventable diseases such as measles, mumps and rubella, a high percentage of the
population needs to be fully immunised to prevent wide-spread community transmission (Table 2).1 With
a basic reproduction number (R0) of 12 – 18, measles is one of the most contagious and communicable of all infectious diseases.1,
15 Influenza and coronavirus disease 2019 (COVID-19) have comparatively lower basic reproduction numbers (Table
1).1, 21
Table 2. Disease transmissibility ratings and herd immunity thresholds required to prevent wide-spread
community transmission.1
| Disease |
Basic reproduction number (R0)* |
Herd immunity threshold |
| Measles |
12 – 18 |
92 – 94% |
| Mumps |
4 – 7 |
75 – 86% |
| Rubella |
6 – 7 |
83 – 85% |
| Coronavirus (COVID-19 |
4.08† |
Unknown |
| Influenza |
1.4 – 4 |
30 – 75% |
*R0 values are estimated values, often deriving from global averages, such is the case with
COVID-19 21. R0 values represent the estimated spreading potential of an infection by calculating
the number of secondary cases that can be infected by one infectious case, within a given, and susceptible population.1 Ranges
can therefore vary greatly between populations depending on the means of calculation and the specific population.21
†The mean R0 number of the delta variant of COVID-19 is estimated to be 5.08 (range 3 – 8)22
First MMR dose now recommended at age 12 months
Following the 2019 measles outbreak, the National Immunisation Schedule was revised on 1 October, 2020, recommending that
children now receive the first MMR dose at age 12 months, and a second dose at age 15 months (previously recommended at
age 15 months and age four years).1 MMR vaccination is fully funded for New Zealand citizens and residents, and
contacts of confirmed cases, including catch-up vaccinations (see Table 3 for recommended MMR vaccination
schedule).1
Encourage immunisation of susceptible individuals
Any person born on or after 1 January, 1969, who has not received two documented doses of the combined MMR
vaccine, is considered susceptible to one or more of measles, mumps and rubella, and includes those who have:1, 16
- Received partial vaccination with one or two doses of a measles-only vaccine (or measles-rubella vaccine
for people vaccinated overseas)
- Clinical history of infection of one or more of the diseases without further documentation of immunity
(see: “Criteria for determining immunity”)
All susceptible individuals should receive one or two doses of MMR vaccine (see Table 3 for dosing
recommendations).1 If vaccination history is not available or is uncertain (i.e. number of doses and/or type
of vaccine), advise patients that there is no safety concern in re-vaccinating with MMR and this is recommended.1 However,
as a live attenuated vaccine, there are certain people for whom MMR is contraindicated (see: “Contraindications
and cautions”).1
Table 3. Recommended MMR immunisation schedule. Adapted from the “Immunisation Handbook” (2020).1
| Patient group
|
Recommended vaccination
(all doses of MMR must be given at least four weeks apart) |
Childhood immunisation schedule for children born in New Zealand |
Two doses of MMR*; dose one at age 12 months and dose two at age 15
months |
Early vaccination for infants during an outbreak |
A single dose of MMR0 [zero] can be given to infants aged between six and 11 months for early
protection
N.B. Any infants receiving the MMR0 vaccine still require two further doses of MMR, as per the usual childhood schedule. |
Catch-up schedule for those born from 1 January, 1969, without documented history
having received two doses of MMR or evidence of serologic immunity against all three diseases |
Two doses of MMR; recommended catch-up doses for patients with vaccination histories
of:
- No prior MMR – two doses
- One MMR – one dose
- Any number of measles, mumps or rubella vaccines (alone or combined, e.g. measles-rubella) and
no MMR – two doses
N.B. Pregnancy should be avoided for at least four weeks following final vaccination. |
Women who are pregnant |
MMR is contraindicated during pregnancy (due to the possibility of fetal harm)
N.B. MMR can be given to women who are breastfeeding. |
Immunocompromised individuals |
MMR is contraindicated; close contacts should be vaccinated (see below) |
People born in New Zealand before 1 January, 1969 |
MMR is not required as they are considered immune to measles (due to presumed exposure to wild-type
measles before the introduction of MMR)
N.B. Those born before 1980 are considered immune to mumps. |
New Zealand population groups at higher risk of infection
In New Zealand, people most at risk of measles exposure and infection include those:1
- Who
are not fully vaccinated with MMR, particularly children aged under two years, and adolescents and young adults,
aged between 15 and 30 years (see: “Adolescents and young adults in New Zealand are under-immunised”)13
-
Returning
from recent travel overseas with uncertain immunisation history, due to higher risk of exposure in measles-endemic
countries
-
Born
in countries where complete vaccination with all three antigens is less likely or access is difficult
- Working
in certain occupations including health care, early childhood education services and other high-contact occupations*,
who are at increased risk of both contracting and transmitting infections
*High-contact occupations include those working in emergency and essential services such as armed
forces, immigration/refugee centres, long-term care facilities, correctional facilities and border workers1
For further information on occupation-related vaccination, see:
www.health.govt.nz/our-work/immunisation-handbook-2020/4-immunisation-special-groups#4-8
MMR immunity gap increases the risk of fetal rubella infection
Although rubella cases have remained low, a large number of women falling within this immunity gap are now of childbearing
age and susceptible to rubella infection, increasing the risk of fetal infection and serious complications such as CRS (see:
“Rubella”).1 Women of childbearing age, especially those who are planning pregnancy, should
be asked if they are fully immunised against rubella with MMR (see Table 2 for recommended vaccination
schedule if catch-up is required).1
N.B. In New Zealand, serological testing for immunity against rubella is typically only performed as part of antenatal
care and is not funded as a precautionary measure for women planning pregnancy.1 If serology results indicate
a lack of immunity during pregnancy, MMR vaccination should be given following delivery.1
Further information on MMR immunisation catch-ups is available from:
www.health.govt.nz/our-work/immunisation-handbook-2020/appendix-2-planning-immunisation-catch-ups
Further information on MMR immunisation for special groups is available from:
www.health.govt.nz/our-work/immunisation-handbook-2020/4-immunisation-special-groups
Scheduling MMR with COVID-19 vaccination
The requirements for spacing between administration of mRNA COVID-19 vaccine with other vaccines, with the exception of
the vaccine for herpes zoster, have been removed. Previously, it was recommended that a gap of two weeks be observed between
COVID-19 vaccination and any non-live vaccine, or four weeks with a live vaccine, such as MMR.1 If a person therefore
requires MMR vaccine and also COIVD-19 vaccine, the advice is that they can now be administered concurrently, and that MMR
can be given either immediately before or after COVID-19 vaccine.1
Further information about COVID-19 vaccination is available from the Immunisation Handbook (see Section 5.4.5
for information on co-administration with other vaccines):
www.health.govt.nz/our-work/immunisation-handbook-2020/5-coronavirus-disease-covid-19
Contraindications and cautions
As MMR is a live vaccine, people with contraindications include those:1
-
Who are pregnant
-
Who are immunocompromised
-
With proven anaphylaxis to either the vaccine itself or a component within it, e.g. gelatin or neomycin.
N.B. People with egg allergies (including anaphylaxis) can safely receive the vaccine.
-
Who have been immunised with another live vaccine* within the previous four weeks; All vaccines
can be given concurrently with MMR vaccine (with separate syringes and different injection sites), however, a four week
gap is required for administration of any other live vaccine if not administered concurrently. There is no longer a requirement
for spacing with inactivated vaccines, e.g. the COVID-19 vaccine.
*Additional live attenuated vaccines include varicella, rotavirus, tuberculosis (BCG) and zoster1
Further information about co-administration of MMR with other vaccines is available from the Immunisation
Handbook (see Section 12.4.4)
www.health.govt.nz/our-work/immunisation-handbook-2020/12-measles#11-6
Two doses are needed for full immunity
Following the second dose of MMR the serologic evidence of immunity increases to 99% against measles, 83 – 88% against
mumps, and is likely higher than 90 – 97% against rubella.1 Almost all people who do not develop protective immunity
following the first MMR dose, do so after the second. Rarely, fully immune people can still contract measles following two
doses, however, it is usually less severe and hospitalisation is less likely.1
Inform patients about potential adverse reactions
Following vaccination, some patients may experience common, mild adverse reactions such as rash, fever, submaxillary gland
swelling or joint pain.1, 23 Mild reactions typically resolve within a few days without treatment. Patients can
manage symptoms by resting, drinking plenty of fluids and relieving fever or discomfort with mild analgesia if required
(e.g. paracetamol or ibuprofen).1, 23 Advise patients to contact a health professional if they are concerned
about troublesome and/or unexpected symptoms.1
N.B. Adverse events following immunisation should be reported to the Centre for Adverse Reactions Monitoring (CARM).1
Vaccination hesitancy
Vaccine hesitancy is a complex barrier to immunisation and refers to a patient or caregiver’s refusal, or
delayed acceptance of vaccination, even when the services are fully funded and available.1, 24 According to a
recently published longitudinal study, over time approximately 30% of the New Zealand population is showing decreasing levels
of confidence in the safety of childhood vaccination.25 Between 1 April, 2020, and 31 March, 2021, the National
Immunisation Register (NIR) reported:7
- 3,290 parents or caregivers (5.2%) had declined/opted out of any one vaccination for their child (i.e.
children turning the milestone age five years during that 12-month period)
- 366 had opted to move their child off the NIR (0.6%)
Vaccine hesitancy is complex and can involve varying factors, including:1, 24
- Vaccine and vaccination-specific concerns; general concern about vaccine safety (i.e. risks/benefits),
associated costs
- Individual and/or social group influences; knowledge, beliefs and attitudes about health and prevention,
perceptions of risks/benefits, personal or anecdotal experience with vaccination, philosophical or conspiratorial beliefs,
complacency
- Contextual issues; culture, religion, socio-economic status, gender, policies and politics, influential
leaders, geographical barriers, historical influences, distrust in health professionals or pharmaceutical industry, media
environment (i.e. ease of access to and abundance of anti-vaccine sentiment)
- Access barriers; physical or geographical access, costs and affordability, ability to understand vaccine
information (i.e. language and/or health literacy)26
Understanding and addressing concerns through effective communication
Clinicians should respectfully correct any misconceptions patients and/or caregivers may have, addressing poor sources
of information with clear, evidence-based research and at the appropriate level of health literacy for the individual.1,
24, 25 Information should include the benefits, risks and possible adverse effects of vaccination, the risks of disease
without vaccination and advice on what they should do if adverse effects occur.1, 25
For further guidance on addressing vaccination concerns, visit:
www.health.govt.nz/our-work/immunisation-handbook-2020/3-vaccination-questions-and-addressing-concerns#3-2
