Published: 10 December 2021
Key practice points:
- Gabapentin and pregabalin, known as gabapentinoids, are funded in New Zealand for use in people with neuropathic pain
and for seizure control in some people with epilepsy
- Gabapentinoids should not be prescribed for people with chronic non-neuropathic pain, e.g. non-specific low back pain,
and are no longer recommended for people with sciatica
- Response to gabapentinoids is variable
- A multidisciplinary approach to managing pain is usually required, combining pharmacological, physical and psychological
treatments to achieve an effective regimen; in some cases, all pharmacological options will be unsuccessful
- Gabapentin and pregabalin have the potential for misuse or diversion; evaluate patients for a history of substance misuse
prior to initiation and monitor for any signs of gabapentinoid misuse
- Regularly review patients to assess treatment response and the continued need for treatment; once pain is stable, attempt
a gradual dose reduction
- A tapering period of at least one week is generally recommended when withdrawing or changing gabapentinoids to minimise
adverse effects; cross tapering, start/stop and taper down and up-titration, are three approaches that can be used to switch
between gabapentinoids
Gabapentin and pregabalin – jointly referred to as gabapentinoids – were originally introduced to manage seizures in people
with epilepsy, however, in practice they have been more widely prescribed for patients with chronic pain, despite mixed
evidence of benefit.
Gabapentinoids are thought to function through stabilising neuronal cell membrane excitability.1 It is possible
that some of their analgesic and anticonvulsant activity derives from activating pre-synaptic calcium channels that are
widely distributed in the body, reducing the release of several neurotransmitters associated with neuropathic pain and seizure
propagation, such as glutamate, noradrenaline, serotonin and dopamine.1, 2
The role of gabapentinoids in neuropathic pain
Neuropathic pain is caused by damage to the somatosensory nervous system which results in painful sensations often described
as burning, shooting or tingling.3 In some people, neuropathic pain is ongoing due to an underlying degenerative
or non-treatable condition, e.g. multiple sclerosis, but in others, improvement is possible or expected, e.g. post-herpetic
neuralgia or post-surgery.
The tricyclic antidepressants (TCAs), amitriptyline and nortriptyline (unapproved indications), and the gabapentinoids
gabapentin and pregabalin, are all first-line pharmacological treatment options for neuropathic pain*.4,
5 Duloxetine is often a first line choice for neuropathic pain in other countries, however, it is neither an approved
nor funded medicine in New Zealand.
*Carbamazepine is recommended as the first-line medicine for people with trigeminal neuralgia5
A meta-analysis (115 studies including 18,087 participants) of first-line medicines used for neuropathic pain found insufficient
evidence that any one medicine was more effective than another (Table 1).4–6 There is some
evidence that efficacy may be improved with combined treatment, for example in people with diabetic peripheral neuropathy,
nortriptyline and pregabalin taken together are more effective at reducing pain than when each medicine is taken alone.3
N.B. Topical capsaicin cream may be considered for people with localised neuropathic pain, e.g. post-herpetic neuralgia
or painful diabetic neuropathy, if oral treatments are not appropriate.5
Table 1: Comparison of the average number needed to treat and average number needed to harm for first-line neuropathic pain
medicines.6 N.B. Other first-line medicines were included in this analysis but are not available in New Zealand or not commonly used for neuropathic pain.
| Medicine |
Daily dose |
Average number needed to treat |
Average number needed to harm |
| Amitriptyline |
25 – 150 mg |
3.6 |
13.4 |
| Pregabalin |
150 – 600 mg |
7.7 |
13.9 |
| Gabapentin |
900 – 3,600 mg |
6.3 |
25.6 |
In mid-2018, the funding for gabapentinoids in New Zealand widened (see: “Pregabalin dispensing
is on the rise”), which has resulted in increased use and growing safety issues.2 The Medicines Adverse
Reactions Committee has expressed concern that gabapentinoids are not being prescribed or taken appropriately.2 Following
a safety review of gabapentin and pregabalin, the Committee suggested that greater awareness is needed
of the potential for people to misuse gabapentinoids, the potential for gabapentinoids to interact with opioids and
of the low-quality evidence for gabapentinoids in treating chronic non-neuropathic pain conditions, e.g. pruritis associated
with end-stage renal disease.2
Adverse effects are common
Problems with balance and sedation are frequently reported: up to one in four patients taking gabapentinoids
experiences sedation and up to 35% experience dizziness or balance difficulties (Table 2).7
Weight gain is likely: Both gabapentin and pregabalin are associated with a variable amount of weight
gain (due to increased appetite and/or oedema), which is dose dependent and typically occurs between 2 – 12 months after
initiation.1 Most patients taking pregabalin maintain their weight within ± 7% of their baseline weight, but
up to 16% can gain ≥ 7% from their baseline.1
Changes in mood may occur: In clinical trials, up to 10% of patients taking pregabalin at therapeutic
doses reported experiencing euphoria; similar effects occur with gabapentin.2, 8 Pregabalin and gabapentin are
anti-epileptic medicines, and anti-epileptic medicines in general have been associated with a small increased risk of suicidal
thoughts and behaviour, regardless of indication.9
Respiratory depression is possible: Evidence shows that in rare cases, gabapentinoids can cause respiratory
depression.2 Lower doses may be necessary in patients at increased risk, including those with respiratory or
neurological disease, renal impairment, older people with frailty and those who take other CNS depressants, e.g. opioids.2
N.B. Pregabalin is currently on the Medicines Monitoring scheme to investigate a potential association with bullous dermatitis
and exfoliating skin reactions.10
For further information on the adverse effects associated with gabapentin and pregabalin, see:
www.nzf.org.nz/nzf_2629 and www.nzf.org.nz/nzf_2631
Table 2: Common adverse effects reported by patients taking gabapentin or pregabalin.1, 4, 7, 8,
11, 12
|
Percentage of patients experiencing adverse effect |
| Dizziness/balance |
13 – 35% |
| Sedation |
11 – 25% |
| Gait disturbance |
14% |
| Weight gain |
Up to 25% |
| Peripheral oedema |
7 – 17% |
| Constipation |
6% |
| Dry mouth |
15% |
| Euphoria* |
1 – 10% |
| Abnormal thinking |
6% |
*In a review of 102 clinical trials of pregabalin, 1 – 10% of patients reported experiencing
euphoria.8 There are no similar clinical trial data for the incidence of euphoria when gabapentin is taken as
prescribed, however, both medicines are misused for their euphoric effects.2 See: “Misuse and
dependence” for further information.
Misuse and dependence
Gabapentinoids may be misused due to their potential to cause euphoria, a state of relaxation and sociability or amplify
the effects of recreational drugs.2 People taking pregabalin are at higher risk of misuse and dependence as it
has greater bioavailability at high doses, a faster onset of action and is more likely to induce euphoria, compared to gabapentin.2 The
risk of misuse is also higher in people who misuse other substances such as opioids or alcohol;2 it is estimated
that among people with an opioid use disorder, up to 68% misuse pregabalin and up to 22% misuse gabapentin.4
The likelihood of gabapentinoid dependence is increased with escalating dose and duration of use;13 concurrent
use of opioids also increases the likelihood of dependence.14
Pregabalin dispensing is on the rise
Since mid-2018, both gabapentin and pregabalin have been available fully funded without restriction.15 Prior to 2018, gabapentin was
funded with Special Authority approval and pregabalin was not funded. Since the funding changes in 2018, the dispensing of pregabalin has increased
significantly, while the dispensing of gabapentin plateaued (Figure 1).16 N.B. Concurrent use of gabapentin and pregabalin
is not funded, i.e. only one medicine will be funded at a time.15
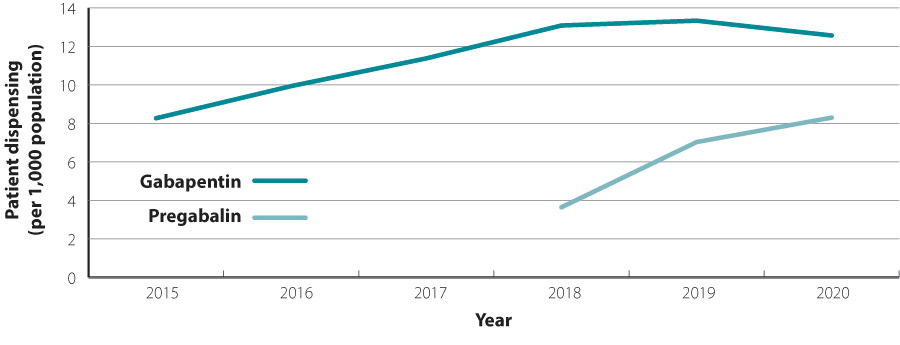
Figure 1: Number of patients per 1,000 dispensed gabapentin and pregabalin in New Zealand from 2015 to 2020.16
N.B. In 2018, both gabapentin and pregabalin were funded without restriction; prior to 2018, gabapentin was funded with Special Authority approval.
TCAs, topical capsaicin and non-pharmacological treatments for neuropathic pain
Before initiating a trial of gabapentin or pregabalin, also consider other possible treatments for neuropathic pain:
- TCAs, e.g. amitriptyline or nortriptyline
(both funded; unapproved indications), may be a good option for pain that is especially troublesome at night or for people
with a history of misuse where gabapentinoids may be inappropriate
- Topical capsaicin cream (0.075% cream funded
by endorsement; 0.025% used for osteoarthritis subject to Special Authority criteria) for people with localised neuropathic
pain who wish to avoid or cannot tolerate oral treatment or when oral treatments are ineffective5
- Non-pharmacological strategies, with or
without pharmacological treatment, such as:3, 5, 17
- Address common psychological co-morbidities, e.g. anxiety and depression
- Physical and psychological interventions, e.g. exercise, physiotherapy, massage, mindfulness, relaxation techniques
and goal setting
- Lifestyle changes to improve overall health, e.g. smoking cessation, healthy eating, reducing excessive alcohol and
illicit drug use
Information for patients about neuropathic pain is available from:
www.healthnavigator.org.nz/health-a-z/n/nerve-pain/
Gabapentin is indicated for the treatment of:9
- Neuropathic pain in adults aged 18 years and older
- Partial seizures with or without secondary generalised tonic-clonic seizures in adults and children aged three years
and older who have not achieved adequate control with standard anti-epileptic medicines
Pregabalin is indicated for:9
- The treatment of neuropathic pain in adults
- Adjunctive treatment in adults with partial seizures with or without secondary generalisation
There is evidence of benefit for people with neuropathic pain who take gabapentinoids, however, response is highly variable
due to multiple factors, e.g. pathophysiology, pain tolerance, genetics, age and renal function.18
Systematic reviews have found moderate quality evidence to support the use of gabapentin and pregabalin in people
with peripheral diabetic neuropathy and post-herpetic neuralgia.4 Approximately 40% of people taking
pregabalin (600 mg, daily) and 30% of people taking gabapentin (≥ 1,200 mg, daily) for at least eight weeks achieved ≥
50% improvement in pain, compared with 10 – 20% of people taking placebo.4
Due to a lack of benefit and evidence of harm, gabapentinoids are not recommended for people with sciatica or
non-specific low back pain;19 evidence for benefit in other types of pain, e.g. cancer-related neuropathic
pain, HIV and episodic migraine, is very limited.4
Gabapentinoids may be considered for unapproved uses but there is limited evidence of their efficacy, and in many cases,
there will be other preferred treatments that are more effective or safer. Use of gabapentinoids for unapproved indications
is “off label” and informed consent should be obtained from patients following a discussion of the risks and benefits.
For further information on the unapproved uses of gabapentinoids, see: nzf.org.nz/nzf_2631
and nzf.org.nz/nzf_2629
Gabapentin and pregabalin should not be used as general analgesics or on an “as needed” basis. They should generally be
avoided or prescribed with caution in people with a history of substance misuse or dependence on prescription medicines.
Gabapentinoids have minimal medicine interactions due to negligible protein binding and no known interference with major
metabolising enzymes, however, they can potentiate the effects of other psychoactive medicines.4
Gabapentinoids should be avoided in people taking CNS depressants, e.g. alcohol or benzodiazepines.1 If
concurrent use cannot be avoided, prescribe lower doses and closely monitor.14
Add opioids with caution. For some people with severe neuropathic pain, first-line medicines will be
insufficient and they may also require an opioid, e.g. tramadol or morphine; there is no evidence that opioids are effective
for long-term pain management.3
When combined with opioids or other sedating medicines, gabapentin increases the likelihood of respiratory depression.2 Gastrointestinal
transit is slowed, raising the bioavailability of gabapentin and higher plasma concentrations are reached; the risk of accidental
overdose is increased and the ability to perform tasks such as driving or operating machinery may be impaired.1, 2 Studies
have shown a greater risk of opioid-related overdose and death (odds ratio 1.49) in people taking a gabapentinoid and an
opioid, compared to an opioid alone.4
For further information on the role of opioids in pain, see:
bpac.org.nz/2018/opioids-chronic.aspx and
bpac.org.nz/2018/opioids.aspx
If pain is still uncontrolled following a trial of non-pharmacological interventions and other suitable analgesics, perform
a risk-benefit analysis to assess if a gabapentinoid is appropriate:
- Consider whether there is evidence that gabapentin or pregabalin is effective for managing the patients specific type
of neuropathic pain 20
- Check other medicines the patient is currently taking; assess for potential interactions, e.g. with CNS depressants 5,
17, 20
- Assess the risk of misuse, dependence or diversion*1, 20
- Consider and discuss potential adverse effects1,17
- Ensure the patient understands the importance of adherence with their regimen, e.g. following instructions for dose
titrations and not taking on an “as needed” basis1, 5
*If there is clinical need to prescribe a gabapentinoid to a patient with a history of misuse,
consider prescribing in short courses, i.e. every seven days rather than the standard 28 days, to monitor and minimise the
risk of misuse
An example of an opioid risk tool that may also be applicable for patients taking gabapentinoids is available
at: www.drugabuse.gov/sites/default/files/opioidrisktool.pdf
Set measurable outcomes for assessing treatment response
As with the treatment of almost any condition, managing patient expectations is key to treatment success, such as in achieving
pain management. Patients may expect complete resolution of their symptoms from pharmacological treatments when, in practice,
only a small reduction in symptom severity (30 – 50% reduction in pain) is achieved and pain is unlikely to completely resolve;
often gabapentinoids reduce pain in a way that only modestly improves quality of life.1
Set measurable and realistic outcomes to assess treatment response, e.g. a reduction in pain score and/or the ability
to perform a task or participate in an activity they could not do before.
A pain diary can help patients assess the effectiveness of treatment, a template is available from:
www.guild.org.au/__data/assets/pdf_file/0023/5945/patient-resource-my-pain-diary-nps-medicinewise.pdf
Select gabapentin or pregabalin – start low, increase dose to effect
Both medicines have similar efficacy, however, in practice, pregabalin may be preferrable for some people as lower doses
can be used , dosing is typically less frequent and the titration period is often faster compared to gabapentin.1 Pregabalin
displays a linear dose-response relationship, where plasma concentrations increase in proportion to increasing dose, whereas
with gabapentin, plasma concentrations do not increase proportionally with the dose.1 N.B. There is limited evidence
to support the preferential use of pregabalin as patient variation, e.g. pathophysiology, pain tolerance, genetics, age
and renal function, greatly influences treatment success.1
Once the decision has been made to initiate a gabapentinoid, take a stepwise approach to pain management:3, 17
- Start low and titrate the dose to achieve maximum benefit or to reach the maximum tolerated dose within dosing recommendations
(Table 3)
- Allow an adequate trial period of at least four weeks or after at least two weeks at the maximum tolerated dose
- Assess response to treatment, e.g. a reduction in pain score and/or the ability to perform a task or participate in
an activity they could not do before
- If response is inadequate or adverse effects are intolerable, consider changing to the other gabapentinoid or a different
class of medicine, e.g. TCAs, or trialling a combination of first-line neuropathic pain medicines (see below)
- Regularly review and re-address lifestyle factors if the pain is inadequately managed or becomes progressively worse
Table 3: Gabapentin and pregabalin dosing protocol for neuropathic pain.9
|
Gabapentin dose (available in funded 100, 300 and
400 mg capsules) |
Pregabalin dose (available in funded 25, 75, 150
and 300 mg capsules) |
General population |
Day 1: 300 mg, once daily
Day 2: 300 mg, twice daily
Day 3: 300 mg, three times daily
Titrate, if required, up to a maximum of 3,600 mg, daily
OR
Day 1: 300 mg, three times daily
Every two to three days thereafter: increase by 300 mg, daily, in three divided doses (i.e. 100 mg per
dose) up to a maximum dose of 3,600 mg, daily |
Day 1: 75 mg, twice daily
Increase, if necessary, after 3 – 7 days to 150 mg, twice daily
Increase further after 7 days, if necessary, to a maximum of 300 mg, twice daily
For divided doses, consider uneven splitting of doses, with a larger dose taken at night |
Frail/older people |
Consider initiating at a lower dose, e.g. 100 mg,
once daily at night; and/or slower titration, e.g. 100 mg, twice daily, then one week later begin
100 mg, three times daily, based on tolerability and response |
Consider initiating at a lower dose, e.g. 25 – 75
mg, once daily; and/or a slower titration based on tolerability and response |
Renal impairment |
Creatinine clearance |
|
|
50 – 80 mL/min |
Reduce dose to 600 – 1,800 mg, daily in three divided doses |
30 – 60 mL/min |
Initially 75 mg, daily; maximum 300 mg, daily in one
or two divided doses |
30 – 49 mL/min |
Reduce dose to 300 – 900 mg, daily in three divided doses |
15 – 29 mL/min |
Reduce dose to 300 mg, on alternate days (up to a maximum dose
of 600 mg, daily) in three divided doses |
15 – 29 mL/min |
Initially 25 – 50 mg, daily; maximum 150 mg, daily in one or two
divided doses |
< 15 mL/min |
Reduce dose to 300 mg on alternate days (up to a maximum dose
of 300 mg, daily) in three divided doses |
< 15 mL/min |
Initially 25 mg, once daily; maximum 75 mg, once daily |
N.B. To reach a dose that is maximally effective, whilst minimising adverse effects, clinicians may prefer to use a slower titration.21
For further information on the dosing regimen of gabapentin and pregabalin, see:
www.nzf.org.nz/nzf_2629 and nzf.org.nz/nzf_2631
Recommended duration of use
As pain reduction is a more realistic expectation than pain elimination, patients are encouraged to continue taking pregabalin
or gabapentin if some benefit is gained, provided the adverse effects and other risks from treatment do not outweigh the
benefit.17
If patients report a partial response to treatment and pain remains problematic, consider adding an additional
first-line neuropathic pain medicine to their regimen, e.g. a TCA.17 In practice, combination treatment may be
more tolerable, as smaller individual doses are often used, however, some people may experience an increase in adverse effects.3,
5, 6
N.B. Concurrent use of gabapentin and pregabalin is not funded.15
For information on alternative medicines for neuropathic pain, see:
nzf.org.nz/nzf_2556#nzf_70735
If treatment has been ineffective or adverse effects are intolerable, gradually discontinue pregabalin
or gabapentin and/or switch to an alternative medicine.17
The effectiveness of pregabalin in the treatment of neuropathic pain has not been assessed in clinical trials for longer
than 12 weeks; the risks and benefits to each patient should be assessed before continuing treatment beyond this point.2
Monitor for adverse effects and manage as required
Adverse effects, e.g. weight gain and sedation, tend to be dose related and transient and often resolve within the first
few weeks to months of initiating treatment.1, 18
Cognitive effects of gabapentinoids, e.g. sedation, dizziness and euphoria. Advise patients to avoid
driving, or operating large machinery until the effect of these medicines is known.1 Consider reducing doses
or switching to a TCA if sedation is intolerable; TCAs also cause sedation, however, these are usually taken once daily
at night.
Monitor patients and encourage them to report any unusual changes in mood, e.g. euphoria, depression or suicidal thoughts/behaviour.18 If
a patient is taking a gabapentinoid and a CNS depressant, e.g. opioids, closely monitor for signs of CNS depression, e.g.
somnolence, sedation or respiratory depression; adjust dose as required.14
Potential for misuse. Primary care clinicians, including community pharmacists, should be
alert for:2, 22
- Early requests for repeats, reports of lost prescriptions or contact with after-hours services for medicine supply
- Patients obtaining prescriptions from multiple doctors
- Escalating doses
Special precautions in older people. Older people who experience dizziness and/or gait disturbances from
taking gabapentin or pregabalin may be at an increased risk of falls;1, 2 adjust dose, as required or discontinue
use.
Assess the goals of treatment regularly and the continued need for medicines
People regularly taking gabapentinoids for neuropathic pain require periodic review. Each review should include
assessment of pain control, dose and the continued need for treatment, patient tolerability, adverse effects and assessment
for misuse and/or dependence.5 Reviews can be conducted:3, 17, 23
- At least four weeks after initiation or after at least two weeks at the maximum tolerated dose
- After four to six weeks of switching to an alternative neuropathic pain medicine
- Monthly in people with a history of misuse
- At least every three months for people concurrently taking opioids
- Approximately every three to six months for other patients taking gabapentinoids long term
After pain is stable, attempt a gradual dose reduction
For patients taking a gabapentinoid long-term, an attempt should be made after six months of responding to treatment,
to gradually reduce the dose or stop treatment, as appropriate, i.e. if pain has resolved.17, 18
Opportunities for a dose reduction or discontinuation may include:23
- Every six months for people taking gabapentinoids long-term
- Inadequate response to treatment
- On request from the patient
- If the patient is experiencing intolerable adverse effects
- If there is evidence of misuse, diversion or non-adherence to the prescribed regimen
- If the patient is pregnant, breastfeeding or planning to conceive (unless the benefits outweigh the potential risk to the fetus or infant)
There are three approaches used to switch between gabapentinoids described in the literature, although efficacy
and safety of these has not yet been established. Clinical judgement – considering individual characteristics, such as age
and tolerability to the original medicine – can help to guide which method is used to switch.21
- Stop/start: Take the last dose of original medicine at night and start the target dose of the new medicine
in the next scheduled dose period (Table 4). Two studies reported this method was effective and well-tolerated.1,
21
- Cross-taper: This involves halving the dose of the original medicine and introducing the new medicine
at half of the intended dose for four days, then stopping the original medicine while continuing with the new at the full
dose.21
- Taper down and stop original medicine, then gradually titrate up the new medicine: Recommended by manufacturers;
a gradual reduction may be more likely to avoid withdrawal symptoms (Table 5).21 N.B. there
may be a loss of analgesia during the tapering down and up-titration phases.21
Switching from gabapentin to pregabalin
Evidence has demonstrated that people who switched from gabapentin to pregabalin (after responding to gabapentin) achieved
greater pain relief and fewer adverse effects, however, people who did not respond to gabapentin and experienced adverse
effects, also experienced adverse effects with pregabalin.1 An example of a direct switch from gabapentin to
pregabalin is shown in Table 4.
Table 4: Conversion dose equivalence estimates for the start/stop approach when switching between gabapentinoids.21
| Gabapentin dose |
Equivalent dose of pregabalin |
| 100 mg, three times daily |
50 mg, twice daily |
| 200 mg, three times daily |
75 mg, twice daily |
| 300 mg, three times daily |
100 mg, twice daily |
| 400 mg, three times daily |
125 mg, twice daily |
| 500 mg, three times daily |
150 mg, twice daily |
| 600 mg, three times daily |
150 mg, morning and 175 mg, night |
| 700 mg, three times daily |
175 mg, twice daily; OR 175 mg, morning and 200 mg, night |
| 800 mg, three times daily |
200 mg, twice daily |
| 900 mg, three times daily |
200 mg, morning and 225 mg, night |
| 1,000 mg, three times daily |
225 mg, twice daily |
| 1,100 mg, three times daily |
225 mg, morning and 250 mg, night |
| 1,200 mg, three times daily |
250 mg, twice daily |
| 1,600 mg, three times daily |
300 mg, twice daily |
N.B. The dose of pregabalin should be reduced in people with an eGFR ≤ 60 mL/min/1.73m2.21
Switching from pregabalin to gabapentin
To reduce the risk of adverse effects, consider titrating down pregabalin, then gradually adding in and titrating up gabapentin
(Table 5).24
Table 5: Example of a dose reduction of pregabalin (150 mg, twice daily) for conversion to gabapentin.24
| Day |
Pregabalin dose |
Gabapentin dose |
| 1 |
150 mg, twice daily |
– |
| 2 |
100 mg, morning and 150 mg, night |
300 mg, night |
| 3 |
100 mg, twice daily |
300 mg, twice daily |
| 4 |
50 mg, morning and 100 mg, night |
300 mg, three times daily |
| 5 |
50 mg, twice daily |
300 mg, three times daily |
| 6 |
50 mg, morning |
300 mg, three times daily |
| 7 |
– |
300 mg, three times daily |
| 8 |
– |
300 mg, morning and afternoon, and 600 mg, night |
A gradual dose taper is required when stopping a gabapentinoid completely
If treatment is inadequate, adverse effects are intolerable or pain has resolved, the gabapentinoid should be stopped
by gradually reducing the dose. Gradual dose tapering allows for the monitoring and minimisation of withdrawal symptoms
such as headache, anxiety, sweating and agitation.1 Factors such as the length of time the gabapentinoid was
taken for, the dose and physiological factors, e.g. age, gender and body weight, influence the duration of dose tapering.18 For
example, if a patient took pregabalin for a short duration at a low dose, withdrawal symptoms should be minimal and a one
week taper period is likely appropriate;18 if a patient has taken a gabapentinoid for longer, e.g. 6 – 12 months,
the taper period should also be longer, i.e. several weeks to months.
To minimise withdrawal symptoms, a suggested regimen is to reduce the daily dose of:23
- Pregabalin at a maximum rate of 50 – 100 mg, per week*
- Gabapentin at a maximum rate of 300 mg, every four days*
*As tolerated; tailored to the patient
Reconsider the diagnosis of neuropathic pain or consider whether an underlying condition is worsening if patients have
trialled multiple first-line medicines without benefit.
A tool to assess the likelihood a patient is experiencing opioid withdrawal may also be useful for patients
withdrawing from gabapentinoids, e.g.: www.mdcalc.com/cows-score-opiate-withdrawal
Clinician’s Notepad: gabapentinoids
Before prescribing
- Consider other
treatments for neuropathic pain, including non-pharmacological strategies (e.g. exercise, physiotherapy, behavioural and
cognitive interventions), and other pharmacological treatments (e.g. TCAs [unapproved indication])
- Perform a risk
benefit analysis
- Is there evidence gabapentinoids are effective for managing the patient’s specific type of neuropathic pain?
- Check for concomitant medicines that may interact, e.g. CNS depressants
- Assess the risk of misuse, dependence or diversion
- Discuss potential adverse effects
- Set measurable
and realistic outcomes to assess treatment response, e.g. a reduction in pain score, or the ability to perform a task or
participate in an activity they could not do before
Prescribing gabapentinoids
- Select gabapentin or pregabalin. Both medicines are equally effective but individual response is variable.
- Lower doses of pregabalin are taken to achieve efficacy equivalent to gabapentin
- Titration of pregabalin may be faster than gabapentin
- Pregabalin is associated with a higher risk of misuse
- Start low and titrate dose to achieve maximum benefit or to reach the maximum tolerated dose. Use lower doses of gabapentin
and pregabalin in people with frailty or renal impairment.
- Allow an adequate trial period of at least four weeks or after at least two weeks at the maximum tolerated dose
- Assess treatment response
- Monitor for adverse effects and manage as required
- Assess the goals of treatment regularly and review the continued need for medicines; after pain is stable, attempt a
gradual dose reduction
Switching or stopping medicines
- Consider switching to or adding another neuropathic pain medicine if initial response is inadequate
- Based on patient tolerability to the original medicine and clinical judgement, a stop/start, cross taper or taper down
and up-titration method can be used to switch between gabapentinoids
- A gradual dose taper is required when stopping a gabapentinoid completely
- Reconsider the diagnosis of neuropathic pain or consider whether an underlying condition is worsening if patients have
trialled multiple first-line medicines without benefit
