Peak bone mass typically occurs around the age of 20 years in women and closer to 30 years in men, after which bone
mineral density progressively declines.1, 2 A reduction in bone mass is a normal part of ageing but several
factors influence bone mass and susceptibility to fractures in later life, including genetic factors, nutrition, body
fat, physical activity, co-morbidities and medicine use, such as oral or high dose inhaled corticosteroids.1, 2 In
women, oestrogen deficiency resulting from menopause causes an accelerated period of bone loss that continues for up to
ten years.1
Osteoporosis is a systemic condition characterised by low bone mineral density (BMD) and disrupted bone architecture,
which increases in prevalence with age. For example, research in Australia estimates a prevalence of osteoporosis of approximately
23% in females aged 50 years and over, increasing to 43% in females aged 70 years and over. Rates in males are lower,
at approximately 6% in those aged 50 years and over, and 13% in those aged 70 years and over.3 There is little
data available on the prevalence of osteoporosis in New Zealand, however, the incidence of hip fractures is estimated
to be lower in older people of Māori, Pacific and Asian ethnicity than in older people of European ethnicity.4
People with osteoporosis have an increased risk of fracture and early mortality
Osteoporosis increases the risk of fracture, most notably in the spine, forearm and hip.1 As older people
are already more prone to falls, osteoporosis drastically increases the probability that a fall will result in fracture
(fragility fracture). Older people with fractures have higher rates of pain, disability, loss of independence, and mortality,
meaning fracture prevention is an important consideration in primary care.5
Various risk factors contribute to an increased risk of fracture independently of BMD, including:6
- Increasing age; age ≥ 65 years for females and ≥ 75 years for males are risk factors
- A history of falls or previous fracture
- Family history of osteoporosis
- BMI < 20 kg/m2
- Current high-dose corticosteroid use ≥ 3 months
- Current smoker
- Early menopause (< 40 years)
- Consumption of more than two alcoholic drinks daily
- Rheumatological and connective tissue disorders; particularly those treated with corticosteroids
Patients with risk factors should have their fracture risk assessed using a clinical tool such as the FRAX or Garvan
hip fracture risk score (see links below) and ideally referred for a DEXA scan (see: “DEXA scanning”).6 FRAX
and Garvan hip fracture risk calculators use age, sex, clinical risk factors and optionally a BMD measurement, to assess
a patient’s risk of fracture. If a DEXA scan is not available or the cost of a scan is a barrier for the patient (if not
covered by ACC), initiation of treatment can be made on the basis of a fracture risk calculation alone.
For fracture risk assessment tools, see:
DEXA scanning
Dual energy X-ray absorptiometry, also known as a DEXA or DXA scan, evaluates BMD at various anatomical locations; the
hip (femoral neck) is used as the preferred site for evaluating fracture risk.5 DEXA scan results are reported
as a T-score, representing the number of standard deviations the patient’s result is above or below the young adult mean
BMD value. Osteoporosis is defined as a BMD 2.5 standard deviations lower than the young adult mean, i.e. a T-score of
–2.5; the risk of fractures increases two-fold for each standard deviation decrease in BMD.5, 6
ACC can fund DEXA scans that relate to an accepted ACC claim for a fragility fracture. If the patient is not eligible
for ACC funding, a cost is associated with the scan that varies by region.6
Treatment should be initiated for patients with a T-score of ≤ –2.5 at the hip or calculated hip fracture risk of ≥
3% in the next ten years (Figure 1). Osteoporosis is defined as a BMD 2.5 standard deviations lower than the young adult
mean, i.e. a T-score of –2.5.5
For all patients, lifestyle modifications should be emphasised to preserve bone health (see: “Lifestyle
and nutrition recommendations for patients at high risk of fracture”). The majority of fractures are caused by a fall.5 Fall
prevention exercise programmes and practical changes around the home should be recommended to reduce risk.
Review the patient’s medicines and assess whether any which increase the risk of falls, such as benzodiazepines or anticholinergic
medicines, could be discontinued or switched for another medicine with less risk.
For further information on falls prevention, see: www.bpac.org.nz/falls/
Bisphosphonates are a first-line pharmacological treatment
Bisphosphonates selectively accumulate within bone where they inhibit osteoclast-induced bone resorption, thereby improving
BMD.1 They are indicated for the treatment and prevention of osteoporosis.7
In post-menopausal females with osteoporosis who have already sustained a low-trauma fracture, treatment with bisphosphonates
reduces the relative risk of an additional fracture by between 30–70% over three to five years compared with placebo.5,
8–10 The NNT over three years to prevent one vertebral fracture is 14–20 patients, and at least 91 to prevent one
hip fracture.11
Bisphosphonates are also recommended for the treatment of osteoporosis in males, but there is less evidence available
regarding their efficacy in preventing fractures. Clinical trials report similar changes in BMD or markers of bone turnover
as seen in females, and reductions in fracture risk.5, 12
Bisphosphonates are available in oral or intravenous forms
Alendronic acid (alendronate), risedronate and zoledronic acid are the first-line bisphosphonates.13 Meta-analyses
suggest that these medicines are similarly effective for reducing the risk of fractures.8, 14 Risedronate
is available fully subsidised without restriction. Special Authority approval is required for patients to qualify for
fully subsidised zoledronic acid and alendronic acid formulations (Table 1). However, from 1 February, 2019 alendronic
acid will be available fully subsidised without restriction.15 Alendronic acid is available as formulations
with or without cholecalciferol.
Check renal function prior to initiation and renewing prescriptions
Bisphosphonates are contraindicated in patients with moderate to severe renal impairment, e.g. < 30–35 mL/min/1.73m2. 7
Table 1. Bisphosphonates for the treatment of osteoporosis* in primary care7
| Route |
Medicine |
Dosing regimen |
| Oral † |
Alendronic acid ‡ |
70 mg, once weekly |
|
Alendronic acid + cholecalciferol ‡ |
70 mg alendronic acid + 140 micrograms cholecalciferol, once weekly |
|
Risedronate |
35 mg, once weekly |
| Intravenous** |
Zoledronic acid ‡ |
One injection of 5 mg/100 mL, 12 monthly (but most commonly given 18–24 monthly)6 |
* For dosing regimens relating to additional indications (e.g. Paget’s disease), see the NZF:
www.nzf.org.nz
† Etidronate is also available as an oral bisphosphonate treatment option. This is an older bisphosphonate medicine with less evidence of effectiveness
than other bisphosphonate medicines.16 It is no longer used in some countries, e.g. the United Kingdom and United States,
and use in New Zealand is no longer recommended.8,17
‡ Subsidised with Special Authority approval. From 1 February, 2019 formulations of alendronic acid will be
available fully subsidised without restriction. The dose of
cholecalciferol in the combined product is sufficient to be used as a supplement if required, however, for patients with more severe deficiency other forms of vitamin
D replacement may be required.7
** Pamidronate is an additional intravenous bisphosphonate, however, it is not indicated for the treatment of osteoporosis.7
Pamidronate and zoledronic acid are not interchangeable.
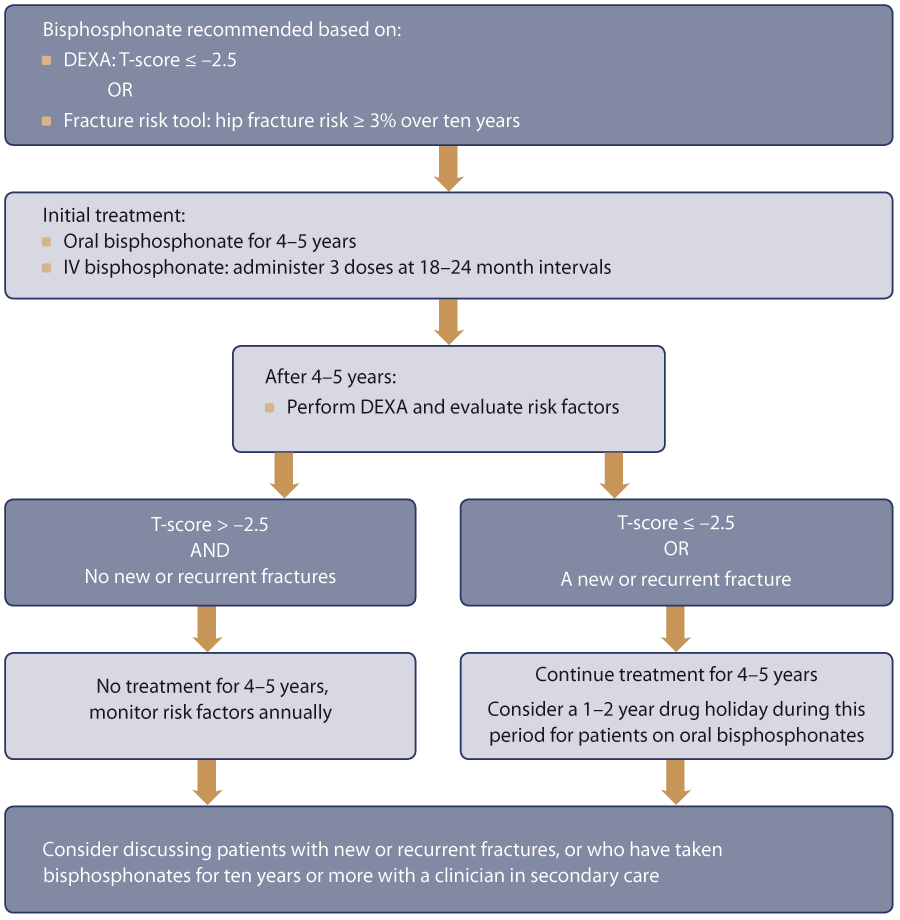
Figure 1. Algorithm for assessing treatment duration with bisphosphonates. Adapted from Osteoporosis New Zealand.6
Adverse effects and ease of adherence can influence which bisphosphonate to prescribe
Specific adverse effects related to each formulation of bisphosphonate may influence which medicine to prescribe.6,
7
Oral bisphosphonates
The main adverse effect of oral bisphosphonates is gastrointestinal irritation, which occurs in 20–30% of patients.7 The
risk can be reduced by taking tablets with water on an empty stomach and then remaining upright for at least 30 minutes.7 Ask
patients to report if they develop more severe gastrointestinal irritation such as dysphagia, heartburn or pain on swallowing
as these symptoms can be indicative of oesophagitis.
Intravenous bisphosphonates
Intravenous infusions of bisphosphonates can cause flu-like symptoms due to an acute phase reaction in approximately
30% of patients after the first infusion.18 Patients can be offered paracetamol or ibuprofen to take shortly
after the infusion to reduce the severity of these symptoms.6
Zoledronic acid is administered no more than once annually, which takes approximately 15 minutes.6 This
option may be simpler for adherence than taking a weekly pill. Intravenous administration may also overcome gastrointestinal
limitations associated with oral regimens. However, infusion-specific adverse effects may still be a barrier to treatment,
not all practices in New Zealand offer zoledronic acid infusions, and this option is likely to be more expensive for patients
due to the additional cost of the infusion procedure. Zoledronic acid is contraindicated in patients with a creatinine
clearance ≤ 35 mL/min.7
Rare adverse effects
Atypical femoral fractures are associated with bisphosphonate use, despite treatment reducing the overall
risk of fractures.9, 19 For example, in 1,000 patients treated with bisphosphonates for three years, 11 hip
fractures are prevented, compared to approximately one case of atypical femoral fracture caused.20 Many atypical
femoral fractures involve minimal or no trauma, and may arise from small stress fractures in the lateral cortex of the
femur that fail to heal, propagating to become complete transverse shaft fractures.9
Best practice tip: advise patients to report any thigh, hip or groin pain during treatment with bisphosphonates.
Osteonecrosis of the jaw has been reported in patients taking bisphosphonates, however, approximately 90%
of cases have occurred in patients taking higher doses used in the treatment of cancer.21 Whether patients
taking bisphosphonates at lower doses for the treatment of osteoporosis are at risk is unclear as reported cases are rare
and osteonecrosis of the jaw can also occur in people who have never used bisphosphonates.21 Regular dental
care and good oral hygiene are recommended to help lower the risk of osteonecrosis of the jaw.22, 23
Best practice tip: advise patients to report any tooth pain, swelling or numbness in their jaw during treatment with bisphosphonates.
For further information on taking bisphosphonates and treatment-specific adverse effects, see:
Patient information leaflets for bisphosphonate medicines are available from the NZF:
www.nzf.org.nz/nzf_70421
Lifestyle and nutrition recommendations for patients at high risk of fracture
Encourage patients to:5, 6, 20
- Engage in regular physical activity and exercises to promote balance, e.g. ≥ 30 minutes of weight-bearing exercise
per day and balance exercises such as Tai Chi or core stability training
- Have a low alcohol intake (≤ two drinks per day; ≥ two alcohol-free days per week)
- Stop smoking
- Maintain a BMI of 20–25 kg/m2 (healthy diet; ≥ 30 minutes weight bearing exercise daily)
- Have adequate sun exposure
- Older people are at an increased risk of vitamin D deficiency and supplementation may be necessary in some cases alongside
an adequate dietary intake of calcium (Table 2).
Best practice tip: Recommending 5–10 minutes’ exposure to sunlight 4–6 times per week can prevent vitamin D deficiency
and therefore alleviate the need for supplementation.6 However, excessive sun exposure should be avoided
to limit the risk of melanoma. Sunscreen should be used, but liberal quantities may reduce vitamin D absorption.
Table 2. Guidance on vitamin D and calcium in patients with osteoporosis5, 31, 32
| Vitamin D |
Calcium |
- Vitamin D supplementation is reserved for older people with frailty* or those at risk of moderate-severe deficiency
- Levels can be deficient in patients who are housebound or in residential or nursing homes as vitamin D is primarily
derived from sun exposure
- Where required, doses of 400–800 IU/day or 1.25 mg/month are recommended ‡
- Testing for vitamin D levels is rarely necessary
|
- Dietary sources of calcium are preferred rather than the use of calcium supplements
- Recommended intake levels are 500–1300 mg, daily, depending on a patient’s sex and age.† This equates to two to
three cups of milk or four to six slices of cheese per day.
|
For more information on vitamin D and calcium supplementation, see “Vitamin D and calcium supplementation in primary
care: an update” at www.bpac.org.nz/BPJ/2016/July/supplementation.aspx.
For dietary sources of calcium that can be discussed with patients, see:
https://nutritionfoundation.org.nz/nutrition-facts/minerals/calcium
* For further information on assessing frailty in older people, see:
www.bpac.org.nz/2018/frailty.aspx
† Guidelines for the management of people with osteoporosis in New Zealand recommend that 500 mg, daily is
sufficient for adults,6 and in the United Kingdom 700–1200 mg, daily is recommended for adults.5 The
New Zealand Nutrition Foundation recommends 1000–1300 mg, daily, for adults.31
‡ Alendronic acid + cholecalciferol can be prescribed for patients requiring a bisphosphonate and vitamin
D supplementation. The dose of cholecalciferol is sufficient to be used as a supplement, however, for patients with more
severe deficiency other forms of vitamin D replacement may be required.7
Guideline groups in various countries, including New Zealand6, the United Kingdom5, the United
States8, 24 and Europe25 have arrived at largely similar recommendations for clinicians treating
patients with osteoporosis on the basis of current evidence. There is sustained fracture prevention for three to five
years of bisphosphonate treatment, however, the risks, albeit small, of rare adverse effects increase with time. There
is currently little evidence to guide prescribing decisions beyond five years of treatment with bisphosphonates, including
the relative benefits and risks of re-initiating bisphosphonates after a ”drug holiday”, and no clinical trials have investigated
use beyond ten years.25 The current state of evidence is likely to remain the case for some time.
New Zealand guidelines recommend that bisphosphonates should initially be taken for:6
- Four to five years of alendronic acid or risedronate
- Three doses of zoledronic acid, at 18–24 month intervals
It is recommended BMD should be reassessed with a DEXA scan after this initial treatment period to help decide, together
with the patient, whether treatment should continue or be paused at this point (Figure 1).
Monitoring during treatment
Measuring markers of bone turnover, such as serum procollagen type I N-terminal propeptide (PINP), is recommended in
some guidelines to assess adherence to oral bisphosphonates, or assess response after treatment is initiated or discontinued.6 There
is currently little evidence available regarding using these measures to guide clinical decisions.26 Some
clinicians in secondary care may request PINP measurements, however, this test is not used often in primary care.
Continuing treatment is recommended in patients at high risk
Patients with high risk criteria should continue treatment with a bisphosphonate for an additional four to five years,
including those:6
- With a femoral T-score of ≤ –2.5
- Who have had a new or recurrent fracture while receiving bisphosphonate treatment
Withdrawing treatment for a one to two year period within this four to five years of additional treatment is recommended
to reduce the risk of atypical femoral fracture (see: “Rare adverse effects”).6
For patients who continue bisphosphonates and have been using them for ten years or more, or who have repeat fractures
despite treatment, discussion with or referral to a clinician in secondary care, such as an endocrinologist or geriatrician,
is recommended.6, 25
Pausing treatment may be appropriate for other patients
For patients who do not meet high risk criteria, pausing treatment may be appropriate (a “drug holiday”) after discussing
with them the benefits and risks of continuing or withdrawing bisphosphonates. Emphasise to patients that they can re-initiate
treatment if their risk changes and that they will be regularly monitored after withdrawal (see below).
Patients receive ongoing benefit from a five-year course of bisphosphonates
Studies have found that increases in BMD at the hip with bisphosphonate use reach a plateau after approximately three
years of treatment.9 If treatment is continued, these increases in hip BMD are maintained and there are ongoing
increases in vertebral BMD. If treatment is stopped after this initial period of bisphosphonate use there is a gradual
reduction in BMD, however, patients continue to receive ongoing benefit. For example, in the FLEX and HORIZON PFT extension
studies (see: “Evidence from longer term trials of bisphosphonates”) patients who were randomised to receive placebo treatment
half-way through the studies still had higher BMD scores at the end of the placebo treatment period than they did at the
start of the study before initiating bisphosphonates.27
The incidence of atypical femoral fractures is very low but may increase with longer durations of bisphosphonate use
A 2016 report from the American Society of Bone and Mineral Research concluded that although findings were variable,
it is likely that a longer duration of use of bisphosphonates leads to a greater risk of atypical femoral fractures, with
incidence increasing from approximately 16 per year for every 100,000 people treated with bisphosphonates for five years,
to 113 per year for every 100,000 people treated with bisphosphonates for ten years.24 Withdrawing treatment
may lead to a reduction in risk; for example, one study combining national prescription and hospital data in Sweden reported
that the risk of atypical femoral fracture declines by 70% per year after stopping bisphosphonates.27
Managing patients who pause bisphosphonate treatment
For patients who discontinue bisphosphonates, conduct an annual review of risk factors. A repeat DEXA scan is recommended
after two to three years.5, 24 On the basis of the annual review or repeat DEXA scan results, clinicians
and patients may decide to re-initiate a bisphosphonate, e.g. if DEXA scan results reveal a T-score of ≤ –2.5, or patients
are exposed to additional risk factors such as initiating long-term corticosteroid treatment.
Annual visits can also be an opportunity to reinforce non-pharmacological approaches to reducing the risk of falls and
improving bone health, such as diet, physical activity and balance exercises.2
Evidence from longer term trials of bisphosphonates
Most clinical trials investigating the use of bisphosphonates have been conducted over periods of three to five years.9 There
is much less evidence to guide prescribing beyond five years of bisphosphonate use.
Two studies have examined the effects of a three to five-year course of bisphosphonate treatment compared to six to
ten years (see below). These studies did not find any additional benefit of longer treatment courses on rates of fracture
overall or non-vertebral fractures (which includes hip fractures). However, there may be benefit of longer courses on
rates of vertebral fracture; for example, the large FLEX study reported an NNT of 34 to prevent one vertebral fracture
over the next five years with alendronic acid treatment.28 In women with T-scores ≤ –2.5 the NNT is lower,
at 17–24.28
The FLEX trial
What did it compare?
The effects of ten years’ use of alendronic acid compared to five years of alendronic acid followed by five years of
placebo treatment, in a total of 1,099 women.
What were the findings?
The study found that BMD and markers of bone turnover were significantly improved in women who had taken alendronic
acid for ten years compared to women using it for five years. Rates of clinical vertebral fractures were also lower (2.4%
compared to 5.3%). However, rates of fractures at other sites were not significantly different between treatment groups.29 A
subsequent analysis reported that continued treatment may reduce rates of non-vertebral fractures in women with T-scores
of ≤ –2.5 who had not previously had a vertebral fracture, however, this analysis was based on a small number of fractures.30
The HORIZON PFT extension trial
What did it compare?
The effects of six years’ use of zoledronic acid compared to three years use of zoledronic acid followed by three years
of placebo treatment, in over 1,200 women.9
What were the findings?
Six years of zoledronic acid treatment resulted in a lower rate of morphometric vertebral fractures, i.e. fractures
detected by radiography, but there were no statistically significant differences between groups in rates of non-vertebral
fractures.9
