Rivaroxaban is a direct oral anti-coagulant (DOAC) that prolongs blood clotting by preventing thrombin generation via
inhibition of factor Xa. From 1 August, 2018, rivaroxaban will be fully subsided without restriction. Currently, rivaroxaban
is only subsidised with Special Authority approval for a short period of prophylaxis following total hip or total knee
replacement. The availability of a third fully subsidised oral anticoagulant means that if treatment with a DOAC is preferable
to warfarin, prescribers will be able to choose between dabigatran and rivaroxaban, depending on the patient’s clinical
circumstances and preference.
Indications for rivaroxaban
Rivaroxaban is indicated for:1
- Prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation (AF) and at least one risk
factor, i.e. congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke or transient
ischaemic attack
- Prevention of venous thromboembolism following knee or hip replacement surgery
- Prevention of recurrent deep vein thrombosis (DVT) or pulmonary embolism (PE)
- The treatment of DVT or PE
Rivaroxaban is contraindicated in patients who:1, 2
- Are actively bleeding or have a high risk of major bleeding
- Have a prosthetic heart valve
- Have moderate to severe hepatic dysfunction associated with coagulopathy
- Have renal dysfunction with a creatinine clearance (CrCl) < 15 mL/min
Rivaroxaban can now be prescribed with caution to people with creatinine clearance of 15-29 mL/min although dose reductions may still
be required in patients with a creatinine clearance < 50 mL/min.2
The “need to know” for prescribing
Rivaroxaban is available in 10 mg*, 15 mg and 20 mg tablets. Rivaroxaban tablets can be placed in blister
packs if required.
The dose of rivaroxaban is determined by indication and renal function (Table 1). Dose adjustments are required in people
with reduced renal function and an assessment of renal function is required in all patients before rivaroxaban is initiated.
Dose adjustment for age alone is not routinely required when prescribing rivaroxaban to older patients.
Rivaroxaban (15 mg and 20 mg tablets) should be taken with food to aid absorption.2 It is not necessary to
take the 10 mg tablets with food.2 There is no listed interaction between rivaroxaban and grapefruit juice,1 but
as rivaroxaban is metabolised by CYP3A4 and grapefruit inhibits this enzyme, it may be a theoretical risk.
Rivaroxaban can be initiated immediately for the treatment of DVT and PE, without the need for prior parenteral anticoagulant
treatment, e.g. low molecular weight heparin, as is required before treatment with dabigatran.1
* The subsidy for the 10 mg formulation is limited to one tablet per day
Manage the risk of bleeding before treatment is initiated
Modifiable risk factors for bleeding should be managed before treatment with any anticoagulant is initiated, e.g. uncontrolled
hypertension, alcohol intake greater than eight standard drinks per week.3 The HAS-BLED prediction tool can
be used to assess bleeding risk in patient with atrial fibrillation.3 A medicine review should be conducted
to determine if the patient is taking any medicines or supplements that may increase their risk of bleeding, e.g. antiplatelets
including aspirin, non-steroidal anti-inflammatory drugs (NSAIDs) or herbal extracts such as garlic, ginkgo or ginseng.3,4
Further information on managing AF, including using the HAS-BLED tool, is available from: “An update on
managing patients with atrial fibrillation”, www.bpac.org.nz/2017/af.aspx
Dose adjustments are required in patients with renal dysfunction
Prior to prescribing rivaroxaban, the patient’s renal function should be calculated using the Cockcroft-Gault equation
to determine if dose adjustments are necessary (Table 1).
A tool for calculating creatinine clearance using the Cockcroft-Gault equation is available from: https://nzf.org.nz/nzf/resource/Creatinine%20Clearance%20Calculator.htm
Table 1: Rivaroxaban dosing as determined by renal function calculated by the Cockcroft-Gault equation
(adapted from NZF and data sheet)1,2
Creatinine clearance (mL/minute) |
Indications |
Prevention of stroke and systemic embolism in non-valvular AF |
Prevention and treatment of DVT and PE |
Prevention of venous thromboembolism following joint replacement surgery |
>49 |
20 mg, once daily |
15 mg, twice daily for 21 days, then 20 mg, once daily for 6–12 months, then
maintain at 20 mg, once daily, or consider 10 mg, once daily, if the risk of bleeding
outweighs risk of recurrent DVT or PE |
10 mg, once daily, starting six to ten hours post-surgery, for two weeks following
a knee replacement or five weeks following a hip replacement |
30–49 |
15 mg, once daily |
15–29
(Use with caution) |
<15 |
Avoid |
Avoid |
Avoid |
N.B. Rivaroxaban should be used with caution in patients with renal impairment if there is concurrent use of medicines that increase the plasma rivaroxaban concentration.
Monitoring patients taking rivaroxaban
Routine testing of anticoagulant effect is not required during treatment with rivaroxaban. Testing may, however, be
required in certain clinical circumstances such as patients with moderate to severe renal dysfunction, prior to surgery
or in the event of bleeding.
Annual testing of renal function is recommended for all patients taking rivaroxaban, as with all anticoagulants.3 More
frequent monitoring may be appropriate in patients with progressive kidney disease or in those with a dehydrating illness,
hypovolaemia or if nephrotoxic medicines are initiated, e.g. NSAIDs. Future dose reductions may be required for patients
with declining renal function after they have begun taking rivaroxaban. If a patient develops acute kidney injury, consider
withdrawing rivaroxaban until renal perfusion has been restored.
For further information on testing and peri-operative management of patients taking rivaroxaban or dabigatran,
see: www.bpac.org.nz/2018/bleeding-guidelines.aspx
Managing missed doses
If a patient taking once-daily rivaroxaban misses a dose, they can take the missed dose later that day, if they remember.
Otherwise, normal dosing on the next day should continue; patients should not take two doses at once.2
If a patient taking twice-daily rivaroxaban (i.e. for treatment or prevention of DVT or PE) misses a dose, the missed
dose should be taken as soon as possible; two doses may be taken at once. Normal dosing should resume the next day.2
An information sheet for patients taking rivaroxaban is available from the NZF: www.mymedicines.nz/home/sheet/Rivaroxaban
Apps for managing patients taking rivaroxaban or dabigatran
Two free applications (“apps”) have been developed by Dr Paul Harper, Clinical Haematologist, MidCentral
DHB to aid clinicians in managing patients taking dabigatran or rivaroxaban. The apps assist in selecting
an appropriate dose for each indication based on the patient’s age and renal function. They also include relevant
medicine information (tablet sizes, pharmacology, storage and advice about taking the medicine), specific dosing instructions
and information about adverse effects, interactions and actions to take if a patient is bleeding.
Download free from the Apple Store:
https://itunes.apple.com/nz/developer/healthobs-ltd/id498413740
Now also available for Android phones: https://play.google.com/store/apps/developer?id=HealthObs+Ltd
In general, the decision of which anticoagulant to choose is made on a case-by-case basis with consideration given to:
- The clinical characteristics of the patient
- The features of the anticoagulant
- The patient’s preferences
- The prescriber’s experience
- Concurrent medicines
The choice between a DOAC or warfarin is largely unchanged
Previously, the choice of anticoagulants has been between warfarin and dabigatran. As rivaroxaban is a DOAC, like dabigatran,
the initial decision of whether a DOAC or warfarin is most appropriate remains largely unchanged. Rivaroxaban provides
many of the same advantages as dabigatran in comparison to warfarin, i.e.:9
- Onset is rapid
- Dosing is standardised (taking into consideration renal function)
- INR testing is not required
- Fewer significant interactions with other medicines and foods
Warfarin, however, is the preferred anticoagulant in patients with:1
- Prosthetic heart valves
- CrCl < 30mL/min; Dabigatran is contraindicated if CrCl < 30 mL/min (except low dose and short-term use following joint replacement surgery).
Rivaroxaban can be used with caution with CrCl 15-29 mL/min but is contraindicated if CrCl < 15 mL/min.
The long half life of warfarin, i.e. 40 hours, may provide more protection against thromboembolism than a DOAC if a
dose is missed, and regular INR testing may encourage adherence. Patients who are concerned about the risk of bleeding
(see below) may find INR testing and the availability of a reversal agent for warfarin in primary care reassuring.9
Bleeding risk may influence the treatment decisions
Rivaroxaban and dabigatran are associated with less risk of intracranial haemorrhage than warfarin but more risk of
gastrointestinal bleeding (see: “The safety and efficacy of rivaroxaban compared to other anticoagulants).10, 11 Intracranial
bleeding is perhaps the most concerning adverse effect of any anticoagulant treatment and the lower risk associated with
dabigatran and rivaroxaban may mean that a DOAC is preferred to warfarin. If a patient has an elevated risk of gastrointestinal
bleeding, warfarin or reduced dose dabigatran, i.e. 110 mg, twice daily, may be the preferred anticoagulants.3
Further information on the use of dabigatran and warfarin is available from: www.bpac.org.nz/2017/anticoagulants.aspx
Managing bleeding in a patient taking rivaroxaban
The clinical assessment of a patient taking an anticoagulant should determine the time of onset and location of
the bleeding, the severity and whether bleeding is ongoing.5 Examine the patient for symptoms and signs
of hypovolaemia, e.g., tachycardia, tachypnoea, hypotension, pallor or cyanosis, and specifically
for gastrointestinal bleeding, e.g. melaena, abdominal pain or swelling. The timing and the size of the last dose
of anticoagulant should also be determined, along with the presence of any concurrent medicines or complimentary treatments
that may exacerbate the bleeding.3 In
general, a low threshold for early discussion with secondary care is recommended for patients
taking anticoagulants where bleeding is suspected.
If bleeding occurs in a patient taking rivaroxaban (or dabigatran) the next dose should be skipped while the patient
is evaluated and stabilised.3 Depending on the location of the bleeding, mechanical compression may be appropriate.
Oral tranexamic acid, 15 mg per kg, three to four times daily, can be used to manage DOAC-associated bleeding.6,7
There
is no reversal agent available for rivaroxaban in New Zealand and management options include
fluid replacement, wound packing, blood transfusion or surgery.
Once a patient has been stabilised, the concentration of rivaroxaban, and its effect, can be expected to have decreased
by more than 90% after four half lives, i.e. approximately 28 hours (range 20 - 36 hours) in
a patient with normal renal function.8
For further information on the management of bleeding in patients taking rivaroxaban or dabigatran, see:
www.bpac.org.nz/2018/bleeding-guidelines.aspx
Deciding if dabigatran or rivaroxaban is the first-choice DOAC
Once it has been established that a DOAC is preferable to warfarin, the next decision is whether to initiate dabigatran
or rivaroxaban (Table 2).
Dabigatran may be preferred when multiple risk factors for bleeding are present
Dabigatran has been associated with a lower risk of major bleeding, compared to rivaroxaban, in observational studies
(see: “The safety and efficacy of rivaroxaban compared to other anticoagulants).12, 13 A reversal agent for
dabigatran, idarucizumab, has been approved for use in New Zealand and is subsidised for use in hospitals.1 There
is currently no reversal agent for rivaroxaban available in New Zealand; Andexanet alfa (Andexxa – coagulation factor
Xa [recombinant] inactivated-zhzo) has recently been approved in the United States to reverse uncontrolled or life-threatening
bleeding in patients treated with rivaroxaban or apixaban.14
Table 2: Clinical scenarios when either dabigatran or rivaroxaban may be preferred
| Dabigatran may be preferred to rivaroxaban when: |
Rivaroxaban may be preferred to dabigatran when: |
Multiple risk factors for bleeding are present, e.g.:3
- Age over 65 years
- Elevated blood pressure
- Previous stroke
- Hepatic dysfunction
- High alcohol intake
|
- Once daily dosing is preferred
- Renal dysfunction is present (although dose adjustments are still required)
- Treating DVT and PE, as low molecular weight heparin is not required
- A history of dyspepsia
- The patient prefers their medicine dispensed in blister packs*
|
* Dabigatran can be blister packaged, but the foil around each individual capsule must remain intact,
therefore it may not fit within a blister pack depending on the number and size of other medicines.
Rivaroxaban generally requires once daily dosing
Once daily dosing for rivaroxaban is sufficient for most indications, which may improve adherence to treatment in comparison
to dabigatran. Treatment adherence to dabigatran is typically high in patients aged over 70 years, but is substantially
lower in patients aged under 50 years, therefore younger patients in particular may prefer the once daily dosing of rivaroxaban.15 However,
treatment adherence is not necessarily just related to frequency of dosing, and this pattern of lower adherence in younger
people may also occur with other anticoagulants.
Rivaroxaban has a half life of five to nine hours in people with normal renal function, but as thrombin generation is
inhibited for 24 hours, a single oral daily dose is sufficient for most indications.16 Twice daily dosing of
rivaroxaban is indicated for the first 21 days of treatment or prevention of DVT or PE.1
The consequences of missing a dose of once-daily rivaroxaban are theoretically greater than missing a dose of twice-daily
dabigatran, as the patient would have declining anticoagulation for a 24 hour period as opposed to a 12 hour period. However,
there is no evidence that this is an issue in practice.
Rivaroxaban may be preferred if renal dysfunction is present
Rivaroxaban is recommended in preference to dabigatran for patients with a creatinine clearance of 30–49 mL/min17
and can be prescribed with caution in patients with a creatinine clearance of 15-29 mL/min.2 This
is because rivaroxaban undergoes substantially less renal excretion (36%) than dabigatran (80%).8 In patients
with moderate CKD, the plasma concentration of rivaroxaban is increased by approximately 50%, compared
to approximately 210% in patients taking dabigatran.8 To account for declining renal function in older patients,
a reduction from dabigatran, 150 mg, twice daily, to 110 mg, twice daily, is recommended in patients
aged 80 years or older.1 Dose
adjustments based purely on age are not required for patients taking rivaroxaban, but dosing based
on renal function is required when rivaroxaban is initiated and regular monitoring of renal function
is recommended.
Rivaroxaban may be better tolerated by patients with a history of dyspepsia
Dyspepsia is reported in approximately 11–12% of patients taking dabigatran,18 whereas dyspepsia associated
with rivaroxaban is reported in 1–10% of patients.2 Although there are no direct comparative studies of
dabigatran and rivaroxaban, this suggests that rivaroxaban may be better tolerated than dabigatran by patients with
a history of dyspepsia. A large retrospective study found that treatment persistence was higher in patients taking
rivaroxaban than in patients taking warfarin or dabigatran, possibly because INR testing was not needed, treatment
was once daily and the rate of dyspepsia may have been lower.19 After two years of treatment, 50% of patients
continued to take rivaroxaban, compared with 31% of patients taking dabigatran and 27% of patients taking warfarin.19 However,
caution is advised when any DOAC is used in a patient with a history of oesophagitis or gastrointestinal ulceration. 20
For other adverse effects, the profile of rivaroxaban and dabigatran are broadly similar with bleeding being the greatest
clinical concern in patients taking either medicine. Nausea and diarrhoea are common adverse effects for both rivaroxaban
and dabigatran.2, 21 Headache, dizziness, hypotension and dermatological symptoms, e.g. rash and pruritus,
are more often reported in patients taking rivaroxaban, than in patients taking dabigatran.2, 21
Medicine interactions with rivaroxaban and dabigatran
As with all anticoagulants, the risk of bleeding is increased by the concurrent use of antiplatelet medicines including
aspirin, however, this may be necessary in some patients, e.g. following an acute coronary syndrome.3 Bleeding
risk is also increased with concurrent use of other medicines that effect platelet function or coagulation such as NSAIDS,
selective serotonin reuptake inhibitors, heparins and some complimentary products. Other medicines increase bleeding risk
or reduce the clinical effect of dabigatran or rivaroxaban by increasing or decreasing their elimination.
The NZF interaction checker can be used to search for potentially significant interactions between dabigatran
and rivaroxaban and other medicines: https://nzf.org.nz
The safety and efficacy of rivaroxaban compared to other anticoagulants
Patients can expect similar levels of protection against stroke and systemic embolism from warfarin, dabigatran or rivaroxaban;
assuming that they are adherent to treatment and that, if they take warfarin, they are able to achieve a time in the therapeutic
range ≥ 70%.3, 11, 12 There are no head-to-head trials comparing rivaroxaban with dabigatran, therefore meta-analyses
of observational studies or indirect comparisons of studies using warfarin as the comparator are the best source of evidence
when assessing the relative benefits and risks of treatment with rivaroxaban.
Rivaroxaban has similar rates of major bleeding as warfarin, but less intracranial bleeding
In patients with non-valvular AF, rivaroxaban is associated with similar rates of major bleeding but decreased rates
of intracranial bleeding, compared with warfarin.10 There is an increased risk of gastrointestinal bleeding
associated with rivaroxaban, compared to warfarin.10, 11
The risk of intracranial haemorrhage in trials comparing rivaroxaban with warfarin for the treatment of venous thromboembolism
has not detected a significantly decreased risk of intracranial haemorrhage associated with rivaroxaban.9 However,
these trials are typically smaller and shorter and a trend for reduced risk of intracranial bleeding is present.9
Rivaroxaban may be associated with a higher risk of bleeding than dabigatran
A large observational study found that rivaroxaban was associated with a higher rate of major bleeding and intracranial
bleeding than dabigatran.12 A meta-analysis of 11 observational studies found that dabigatran was associated
with a statistically significant 21–26% lower risk of major bleeding than rivaroxaban.13
Rivaroxaban has not been associated with an elevated risk of myocardial infarction
Rivaroxaban may be preferred over dabigatran for patients who have an increased risk of myocardial infarction. A meta-analysis
of 12 trials found that dabigatran was associated with an increased risk of myocardial infarction, compared with warfarin.24 Rivaroxaban
was not associated with an increased risk of myocardial infarction in a meta-analysis of nine trials.25
Patients without prosthetic heart valves who are taking warfarin may benefit from switching to a DOAC if their INR results
are frequently outside of the therapeutic range. Patients who develop intolerable adverse effects with warfarin or dabigatran
may wish to switch to rivaroxaban. When switching a patient between oral anticoagulants it is essential that they understand
they should no longer take the original anticoagulant. Asking the patient to bring any remaining medicine to the pharmacy
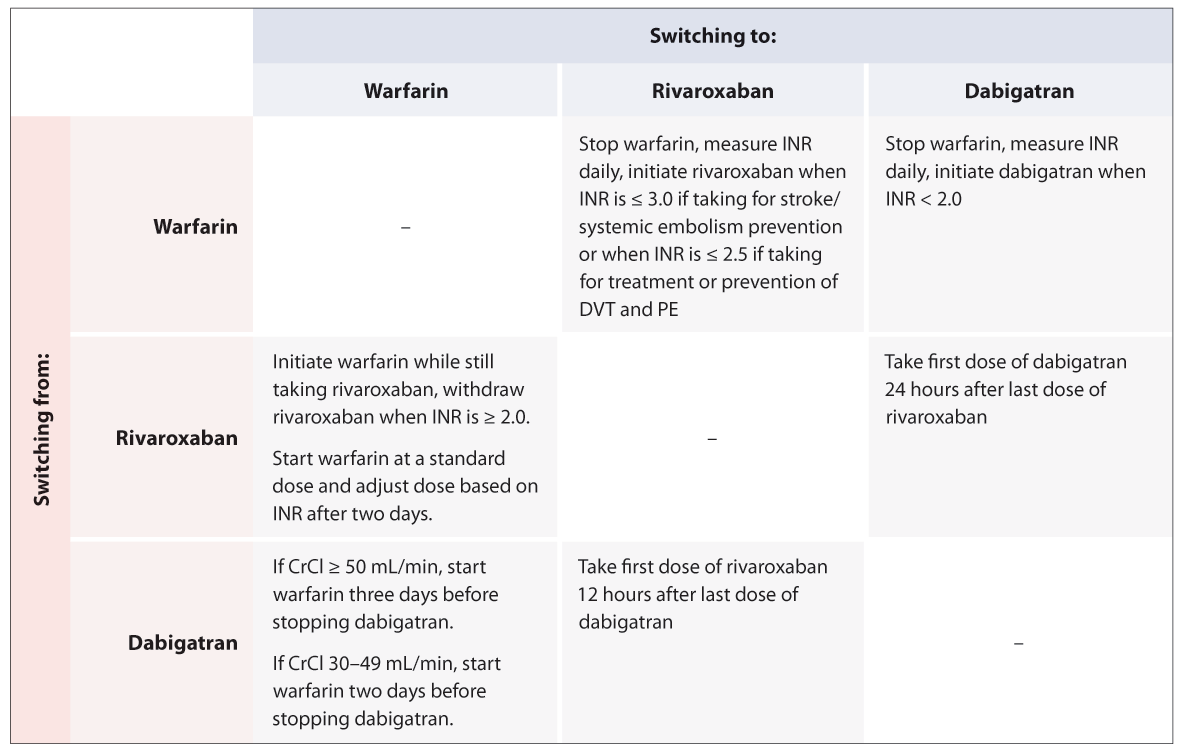
for disposal may help to avoid confusion. Table 3 gives a quick reference guide to switching between anticoagulants.
Switching to rivaroxaban from warfarin
A switch from warfarin to rivaroxaban (or dabigatran) may be appropriate for patients with INR values that are often
outside of the therapeutic range. For example:22
- Two INR values less than 1.5 in the previous six months
- Two INR values greater than five or one value higher than eight in the previous six months
- Less than 65% of the time within the therapeutic range
There is unlikely to be any clinical benefit in switching patients from warfarin to a DOAC if they spend ≥ 70% of the
time within the therapeutic range, as their risk of stroke or systemic embolism is unlikely to be reduced.3 However,
there may be additional reasons that the patient wishes to switch treatment, e.g. inconvenience of INR testing. Intolerable
adverse effects and medicine interactions may also influence the decision to switch from warfarin to a DOAC.
For patients taking warfarin for the prevention of stroke and systemic embolism, warfarin should be stopped,
an INR taken daily, and rivaroxaban initiated when their INR is ≤ 3.0.2, 23
For patients taking warfarin for the treatment and prevention of DVT and PE, warfarin should be stopped,
an INR taken daily, and rivaroxaban initiated when their INR is ≤ 2.5.2, 23
Once the patient has begun taking rivaroxaban, the INR is not a reliable measure of the anticoagulant effect of rivaroxaban.
Switching to warfarin from rivaroxaban
Patients with declining renal function or those who have experienced persistent adverse effects with DOACs may benefit
from switching treatment from rivaroxaban to warfarin.
To ensure anticoagulation is adequate, warfarin and rivaroxaban should be taken concurrently and rivaroxaban withdrawn
when the patient’s INR is ≥ 2.0.2 Warfarin initiation is recommended at a standard dose and after two days,
treatment should be guided by INR testing.2 However, rivaroxaban may continue to contribute to an elevated
INR until 24 hours after the last dose.2
Switching to rivaroxaban from dabigatran
Patients who develop intolerable adverse effects while taking dabigatran, e.g. dyspepsia, or who find it difficult to
adhere to the twice daily dosing of dabigatran, may benefit from switching to rivaroxaban.
It is recommended that patients take their first dose of rivaroxaban, 12 hours after their last dose of dabigatran to
ensure that adequate anticoagulation is maintained.
Table 3: Quick reference guide for switching between anticoagulants