Published: 20 January 2017 | Updated: 25 July 2024 |
What's changed?
25 July 2024 The BPAC Clinical Solutions decision support module on acne management is no longer available.
Funding information, contraindications and cautions have also been updated.
What do prescribers need to know?
- Oral isotretinoin is a highly effective treatment for patients with acne that is causing scarring or distress,
or that persists following other treatments
- Low-dose isotretinoin, e.g. 10 mg per day, is effective for most patients
- Treatment should continue for at least three to four months after acne has cleared to reduce the risk of relapse
- The time for acne clearance to occur is variable and some patients require a longer period of treatment
- If pregnancy is possible, women should use effective contraception, i.e. two methods (refer below), for at least
one month before beginning treatment, during treatment, and for at least one month after stopping
treatment
Isotretinoin is an isomer of retinoic acid that has been used for the treatment of acne for over 30 years.1 Isotretinoin
is recommended for patients with moderate acne that produces scarring or distress, or for acne that persists following
other treatments.2
Treatment with isotretinoin results in decreased sebum production. This reduces acne, prevents scarring and may lead
to a better quality of life and improved mental health in some patients.2 Isotretinoin is funded subject
to Special Authority approval; vocationally registered general practitioners or nurse practitioners working in a relevant
scope of practice are able to prescribe provided they have up-to-date knowledge of the safety issues.
Contraindications and cautions for isotretinoin use
Isotretinoin is highly teratogenic and is contraindicated in women if pregnancy is possible unless effective contraception
is used (Table 1).1 Women who are breastfeeding should avoid using isotretinoin as should
patients with hepatic impairment.1 Hyperlipidaemia is considered a relative contraindication. Patients with
elevated lipids can usually be managed with close monitoring. Advise patients to avoid donating blood during treatment
and for at least one month after treatment has finished.1
Table 1: Contraindications and cautions for treatment with isotretinoin.1
| Contraindications |
Cautions |
- Pregnancy – highly teratogenic (see below)
- Breastfeeding – potential toxicity
- Hepatic impairment – further impairment may occur
- Hyperlipidaemia – can usually be managed with close monitoring
- Concurrent use of tetracyclines – due to the risk of intracranial hypertension
- Hypervitaminosis A – although rarely seen
|
- Diabetes – elevations in fasting blood sugar levels may occur
- Elevated serum triglycerides – risk of pancreatitis
- Prior history of mental illness (see below)
- Dry eye syndrome – risk of keratitis
- Avoid topical keratolytic and exfoliants
|
Further information on isotretinoin is available from the New Zealand Formulary (NZF):
www.nzf.org.nz/nzf_6452
Prescribe low-dose isotretinoin to most patients with acne
The approved dose of isotretinoin is calculated according to the patient’s weight, i.e. 500 micrograms per kg, daily
(in one to two divided doses) for two to four weeks, increased if necessary to 1 mg per kg daily, for 16 – 24 weeks; maximum
cumulative dose 150 mg per kg, per course.1 If a relapse occurs, treatment can be repeated if at least eight
weeks have passed since the previous course.1
In practice, lower doses of isotretinoin are prescribed to the majority of patients in New Zealand (see: “Low-dose
versus weight-based dosing of isotretinoin”), e.g. 10 mg per day until acne has cleared and
for another three to four months thereafter.3 For many patients, a lower dose of 5 mg per day is
likely to be effective.3
To improve absorption and reduce fluctuations in systemic availability, isotretinoin should be taken with or just
after food.4
Low-dose isotretinoin is as effective as weight-based isotretinoin
Low-dose regimens of isotretinoin are generally considered to be as effective as weight-based dosing regimens. A
retrospective review of 1,453 patients treated with isotretinoin over a six-year period concluded that continuing treatment
for at least two months after acne had completely resolved was more important than the dosing regimen in determining
treatment success.5 A low-dose treatment regimen may be better tolerated as the majority of the adverse
effects of isotretinoin are dose-dependent.5
For additional information, see:
www.goodfellowunit.org/gems/acne-low-dose-isotretinoin-10-mg-daily-effective-fewer-side-effects
Low-dose versus weight-based dosing of isotretinoin
Previously, treatment with isotretinoin could only be initiated by dermatologists. In 2008, 8400 patients began treatment
with isotretinoin in the community (Figure 1) with approximately equal numbers of patients prescribed
low-dose and weight-based treatment regimens. In 2009, general practitioners became able to prescribe fully subsidised
isotretinoin to improve access to people living in less affluent areas. Almost 10 000 patients in the community began
treatment with isotretinoin, 54% of whom initially received a low-dose regimen.12 In 2015,
over 13 000 patients began treatment with isotretinoin in the community and 80% of patients received a low-dose regimen.12
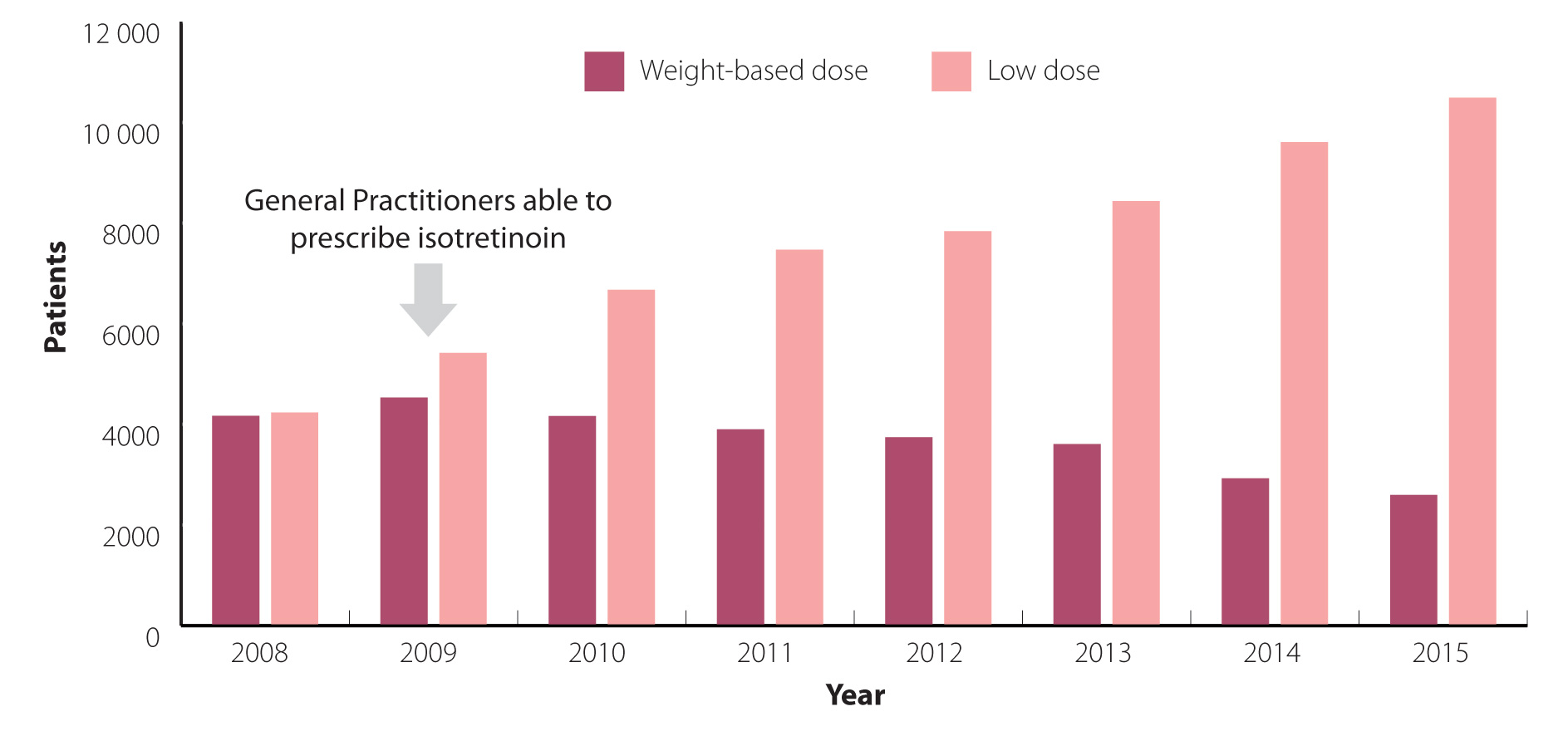
Figure 1: Number of patients initiated isotretinoin at > 20
mg per day (weight-based) or ≤ 20 mg per day (low-dose) from community pharmacies in New Zealand (2008 – 2015).12
N.B Dosing of isotretinoin at ≤ 20 mg per day may also occur due to weight-based calculations in a
small group of patients who weigh 40 kg or less.
Less frequent dosing can reduce the risk of acne flares
Paradoxically, flares of acne are frequently reported by patients after three to six weeks of treatment with higher
doses of isotretinoin.6 Flares of acne are less common in patients taking low-dose isotretinoin.3 If
a patient is likely to experience flares of acne (see below), consider prescribing 10 mg isotretinoin, two to three
times per week, rather than daily. The frequency can then be increased to daily, if tolerated, after four weeks. Expert
opinion is that flares of acne are more common in patients with a large number of macrocomedones (facial closed comedones
larger than 2 – 3 mm in diameter) or very severe acne.
To decrease flares, expert opinion is that patients with
particularly inflammatory acne can be prescribed trimethoprim,
300 mg per day, for the first six to eight weeks of treatment with isotretinoin.
The time taken for acne to clear varies
Patients with acne that is likely to take longer to clear should be advised of this before starting isotretinoin
so that treatment is not stopped if results do not occur as quickly as the patient expects. Experience suggests that
females with acne limited to the face are likely to respond to isotretinoin treatment within three months. Males with
acne on their trunk or back may take five to eight months to respond to treatment with isotretinoin. Patients with
macrocomedonal acne may require as long as 12 to 18 months of treatment with low-dose isotretinoin. Additional risk
factors for a slow response to treatment include: age under 14 years, age over 25 years in females, severe acne, current
smoking and polycystic ovary syndrome.6
Further information and images of macrocomedonal acne is available from:
www.dermnetnz.org/topics/comedonal-acne/
Why is low-dose isotretinoin an unapproved regimen?
The New Zealand recommendations for isotretinoin are based on early studies.5 To determine optimal dosing,
patients were prescribed doses ranging from 0.1 mg per kg, daily, to 1.0 mg per kg, daily.6 The same degree
of efficacy was reported across this range, however, higher daily weight-based doses were interpreted as having lower
rates of relapse.2 This led to the approved dose of isotretinoin of 0.5 – 1 mg per kg, daily. These studies,
however, only compared doses for a fixed time period.5
It is now known that lower doses of isotretinoin, given for longer periods of time, are similarly effective with
a lower risk of adverse effects to the patient. This has led to patients taking lower daily doses of isotretinoin,
i.e. off-label prescribing, for longer periods of time.5
Further information on the unapproved use of medicines is available from:
www.bpac.org.nz/BPJ/2013/March/unapproved-medicines.aspx
Taking isotretinoin during key developmental periods is detrimental. If a woman who is pregnant takes isotretinoin
during weeks six to ten of gestation major disruptions to organogenesis can occur.8 Isotretinoin adversely
affects 25–40% of foetuses exposed during embryogenesis.9
Extreme care is required when prescribing to women of child-bearing age
Effective contraception must be used for at least one month before beginning treatment, during treatment,
and for at least one month after stopping treatment.1 Barrier methods of contraception should not be used
alone and as oral progesterone-only contraceptives need to be taken within a three-hour window these are not recommended.1 Ideally,
two forms of contraception should be used, i.e. condoms and hormonal treatment.1
Begin treatment with isotretinoin on day two or three of the woman’s menstrual cycle.1 Testing
to exclude pregnancy, preferably a serum HCG test, is recommended up to three days prior to treatment, every month
during treatment and five weeks after stopping treatment.1
Retinoic acid is a metabolite of vitamin A which can cause similar adverse effects as excess vitamin A. These include
dryness of the skin, lips (cheilitis – which may persist for months), eyes, nasal and pharyngeal
mucosa.1, 2 Adverse
effects are more likely to be experienced by patients taking higher doses of isotretinoin. A review
of patients taking high-dose isotretinoin (> 0.75 mg per kg, daily) found that cheilitis (96%), eczema (16%) and
tiredness (18%) were frequently reported but these were less common in patients taking low-dose isotretinoin
(< 0.25
mg per kg, daily).10 Myalgia,
arthralgia, changes in vision and headache may also be reported.1 Photosensitivity reactions can occur and
patients taking isotretinoin should be advised to be “sun smart” and to avoid the use of sunbeds.1 The adverse
effects of isotretinoin usually resolve completely once treatment is withdrawn.2
Changes in mood, depression and suicide have been reported in patients taking isotretinoin and monitoring patients’
mood is recommended (see below).2 At a population level, however, studies do not support an association
between treatment with isotretinoin and adverse psychiatric effects.2 Conversely, there is evidence suggesting
that for some patients with acne, treatment may lead to improvements in mood, memory and higher-level cognitive functions.2
There is mixed evidence of an association between the use of isotretinoin and inflammatory bowel disease (IBD). Several
studies are reported to have shown a link between patients taking isotretinoin and subsequent development of IBD, in
particular ulcerative colitis. However, more recent analyses have not confirmed this association.2
Monitoring hepatic function and serum lipids
Isotretinoin treatment can cause dose-related elevations in serum transaminases, cholesterol and triglycerides.2
,4 Current monitoring recommendations (including an electronic decision support tool) for patients taking isotretinoin
include an assessment of hepatic function and serum lipids, before treatment is initiated, after starting treatment
and every three months thereafter.1
In practice, however, there is likely to be little value in routinely monitoring hepatic function and serum lipids
in otherwise healthy patients taking low-dose isotretinoin. In 2016, a systematic review of studies including patients
taking high-dose isotretinoin (≥ 40 mg, daily or ≥ 0.5 mg per kg, daily) found that while treatment with isotretinoin
was associated with changes in serum transaminases and lipids, especially triglycerides and total cholesterol, there
was no evidence to support monthly testing in otherwise healthy patients.11
A pragmatic approach would be to ensure there is a recent assessment of the patient’s hepatic function and lipid
profile and to monitor patients with risks factors, e.g. a history of either hepatic dysfunction or hyperlipidaemia.
Isotretinoin dosing should be reduced or treatment withdrawn in patients with persistently raised serum lipids, or
transaminase,1 e.g. ALT greater than three times the upper limit of normal. Discussion with a dermatologist
is recommended for patients with significantly elevated serum triglycerides; levels > 9 mmol/L have been associated
with acute pancreatitis.1
Advise patients to report adverse changes in mood
When discussing isotretinoin treatment ask how the acne is affecting the patient’s mood and consider their prior
mental health. Inform the patient that there have been reports of depression in patients taking isotretinoin and ask
them to report any adverse changes in mood.
Patients who experience a relapse of acne may be offered a second course of isotretinoin eight weeks after treatment
is completed;1 this can be expected in approximately 22% of patients.5 Patients with risk factors
for acne that is slow to clear are also more likely to experience a relapse as are those who discontinue treatment
before their acne has cleared and those with excessive seborrhoea after treatment has finished.6 Extending
the treatment period to four to six months after acne has cleared may reduce the risk of relapse for these patients.
Consider discussing patients with a dermatologist if they experience multiple relapses of acne despite being adherent
to treatment.
Māori and Pacific peoples with acne may be under treated
There is no evidence that Māori or Pacific peoples have lower rates of acne.13 It is therefore reasonable
to expect that Māori and Pacific peoples would be prescribed isotretinoin at rates similar to that of New Zealand Europeans.
However, analysis of New Zealand prescribing data from 2008 to 2015 suggests that Māori and Pacific peoples may not
be receiving equitable treatment for acne compared to New Zealand European and Asian people (Figure 2).
It is not known if these differences in prescribing are due to under treatment, disparities in access to care, personal
attitudes to acne or a combination of factors. Clinicians in primary care can help to improve acne treatment rates
among Māori and Pacific peoples by discussing acne with all patients who are affected and ensuring patients know that
treatment with isotretinoin is available.
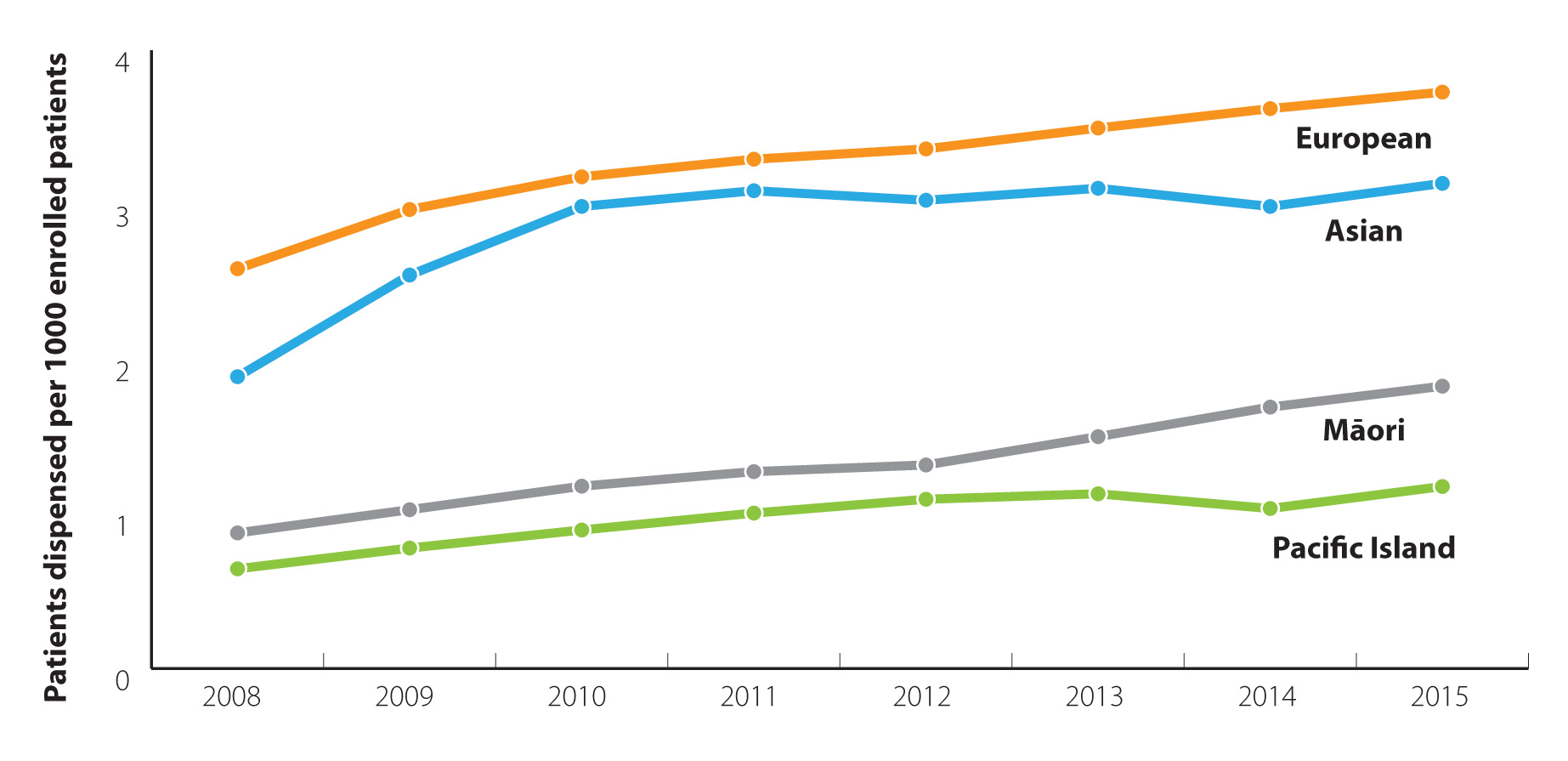
Figure 2: Number of patients dispensed isotretinoin, per
1000 enrolled patients, from community pharmacies in New Zealand by ethnicity (2008 – 2015).12
Isotretinoin capsules and peanut allergy is unlikely to be a concern

Isotretinoin contains soy bean oil which in theory can cause serious reactions in patients with peanut allergy.
The British Association of Dermatologists, however, advises that it would be an “exceptionally” rare event for a patient
with peanut allergy to have a cross-reaction to soy proteins in soy oil.7 Soy products are widely used
in foods and hypersensitivity in a patient is likely to have been previously identified. Furthermore, soy bean oil
in pharmaceuticals is usually refined or hydrogenated and very unlikely to be as allergenic as soy bean protein.
Acknowledgement
Thank you to Associate Professor Marius Rademaker, Clinical Director of the Dermatology Unit, Waikato,
DHB, for expert review of this article.
A webinar on prescribing isotretinoin for acne in primary care presented by Associate Professor Rademaker
is available from the Goodfellow Unit: https://www.goodfellowunit.org/events-and-webinars/prescribing-isotretinoin-acne-primary-care
References
- New Zealand Formulary (NZF). NZF v55. 2016. Available from: www.nzf.org.nz (Accessed
Dec, 2016)
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad
Dermatol 2016;74:945–973.e33. http://dx.doi.org/10.1016/j.jaad.2015.12.037
- Rademaker M, Wishart JM, Birchall NM. Isotretinoin 5 mg daily for low-grade adult acne vulgaris--a placebo-controlled,
randomized double-blind study. J Eur Acad Dermatol Venereol JEADV 2014;28:747–54.
http://dx.doi.org/10.1111/jdv.12170
- Mylan New Zealand Ltd. New Zealand data sheet: Isotane. 2015. Available from:
www.medsafe.govt.nz/profs/datasheet/i/isotanecap.pdf
- Rademaker M. Making sense of the effects of the cumulative dose of isotretinoin in acne vulgaris. Int J Dermatol
2016;55:518–23. http://dx.doi.org/10.1111/ijd.12942
- Rademaker M. Isotretinoin: dose, duration and relapse. What does 30 years of usage tell us? Australas J Dermatol
2013;54:157–62. http://dx.doi.org/10.1111/j.1440-0960.2012.00947.x
- British Association of Dermatologists. Alitretinoin. 2015. Available from:
www.bad.org.uk/shared/get-file.ashx?id=3659&itemtype=document
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008;134:921–31.
http://dx.doi.org/10.1016/j.cell.2008.09.002
- Prevost N, English III JC. Isotretinoin: Update on Controversial Issues. J Pediatr Adolesc Gynecol 2013;26:290–3.
http://dx.doi.org/10.1016/j.jpag.2013.05.007
- Rademaker M. Adverse effects of isotretinoin: A retrospective review of 1743 patients started on isotretinoin.
Australas J Dermatol 2010;51:248–53. http://dx.doi.org/10.1111/j.1440-0960.2010.00657.x
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory Monitoring During Isotretinoin Therapy for Acne: A Systematic
Review and Meta-analysis. JAMA Dermatol 2016;152:35–44.
http://dx.doi.org/10.1001/jamadermatol.2015.3091
- Ministry of Health. Pharmaceutical Claims Collection. 2016.
- Moodie P, Jaine R, Arnold J, et al. Usage and equity of access to isotretinoin in New Zealand by deprivation and
ethnicity. N Z Med J 2011;124:34–43.





