Key practice points:
- Ferric carboxymaltose is an intravenous iron preparation that can be delivered by slow push injection or as an infusion
diluted in saline
- Doses of up to 1000 mg of elemental iron can be administered in one session, taking up to 15 minutes, followed by
30 minutes of observation. Doses over 1000 mg should be divided and administered over two sessions,
at least seven days apart.
- Ferric carboxymaltose can be prescribed by general practitioners, fully subsidised with Special Authority approval,
for patients who have previously trialled oral iron supplements or who require rapid correction of iron levels,
if their serum ferritin is ≤20 micrograms/L
- Intravenous iron is the preferred form of iron supplementation for some patients with co-morbidities such as chronic
kidney disease, symptomatic heart failure or inflammatory bowel disease. If these patients do not meet the
criteria for prescribing by a general practitioner as above, they can be prescribed ferric carboxymaltose, subsidised
with Special Authority approval, on the recommendation of an internal medicine physician, obstetrician, gynaecologist
or anaesthetist.
For guidance on diagnosing and managing iron deficiency anaemia, see: “Anaemia on full blood count:
investigating beyond the pale” www.bpac.org.nz/BT/2013/September/investigating-anaemia.aspx
Anaemia is defined as a decrease in circulating red blood cells or haemoglobin concentration, which affects the body’s ability to transport and utilise oxygen,
causing symptoms such as fatigue and difficulty concentrating.1 There are a wide range of potential causes of anaemia; the most common is iron deficiency.2
Laboratory investigation results consistent with iron deficiency anaemia include low serum ferritin, low transferrin saturation, low iron and increased
total iron binding capacity or serum soluble transferrin receptor.3
Oral iron supplementation is still first-line for many patients
Dietary changes or oral iron supplementation are usually the first-line approach for treating patients with iron deficiency
anaemia in primary care, such as females with heavy menstrual blood loss or patients with low dietary iron intake who
are otherwise healthy.
However, for some patients, oral iron supplementation will not result in a sufficient improvement in iron stores, or
it will cause intolerable adverse effects, such as gastrointestinal symptoms, necessitating a parenteral treatment approach.
Intravenous iron is the preferred approach for some patients with co-morbidities
Intravenous iron supplementation is regarded as the first-line treatment for iron deficiency anaemia in some patients
with inflammatory bowel disease, chronic kidney disease or heart failure. Evidence shows that certain patients will experience
better outcomes with parenteral compared to oral iron supplementation. This includes patients with:
- Clinically active inflammatory bowel disease; intravenous iron produces a faster response and is better tolerated than
oral iron supplementation.4 Oral iron supplementation may still be suitable for patients with controlled
symptoms and only mild anaemia.4
- Chronic kidney disease (CKD) and receiving erythropoietin or undergoing renal dialysis; intravenous iron treatment is
preferred as oral iron supplementation does not supply sufficient iron to the bone marrow to support erythropoiesis. Oral
iron supplementation is suitable for other patients with CKD.5
- Heart failure with reduced ejection fraction; these patients have a worse prognosis if they also have iron deficiency,
therefore guidelines recommend intravenous iron to alleviate symptoms, improve exercise capacity and quality of life.6
See below for details on how these patients may be eligible for subsidised prescription of ferric carboxymaltose.
Intravenous iron provides quicker benefit for patients with severe anaemia
Intravenous iron treatment is appropriate for patients who initially have severe anaemia as it produces a quicker improvement
in the first weeks of treatment than oral iron supplementation.3 This may include patients at risk of cardiovascular
instability due to the degree of their anaemia or who have severe symptoms of fatigue.
Ferric carboxymaltose has now been added to the Community Pharmaceutical Schedule and can be prescribed for a range
of patients with iron deficiency anaemia, subsidised subject to Special Authority approval (Figure 1). Previously, iron
polymaltose was the only subsidised parenteral option that could be prescribed to patients in primary care.
Ferric carboxymaltose Special Authority application: www.pharmac.govt.nz/2017/12/01/SA1675.pdf
General practitioners who wish to offer intravenous iron treatment can prescribe subsidised ferric carboxymaltose to
patients who have trialled oral iron and either do not tolerate it or do not achieve a sufficient improvement, if the
patient’s ferritin level is < 20 micrograms/L (Figure 1). Patients with a serum ferritin level above 20 micrograms/L
can receive subsidised treatment on the recommendation of an internal medicine physician, obstetrician, gynaecologist
or anaesthetist (Figure 1). This could be arranged by phone, email or under a standing order from the relevant physician,
where ferric carboxymaltose is administered in primary care when a patient’s red blood cell indices fall below an agreed
value.
Patients with symptomatic heart failure, active inflammatory bowel disease or chronic kidney disease stage three or
more, where a trial of oral iron supplementation is unlikely to be successful, can now be administered subsidised ferric
carboxymaltose in the community on the recommendation of a relevant specialist as above
Ferric carboxymaltose is likely to be the preferred option for most patients requiring parenteral iron
supplementation as it can provide a higher dose of iron administered in a shorter timeframe. Ferric carboxymaltose is
approved for intravenous bolus use and intravenous infusion, but not intramuscular injection. Iron polymaltose is only
approved for intramuscular injection.7
Special Authority criteria for prescribing subsidised ferric carboxymaltose in the community
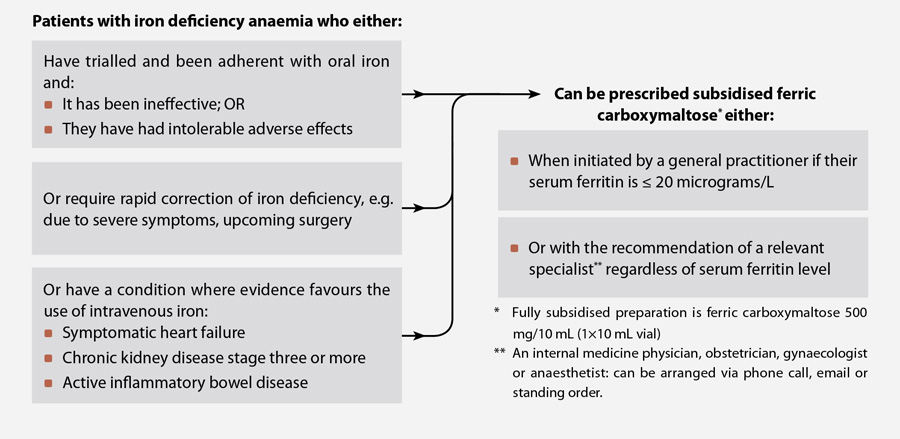
Figure 1: Special Authority criteria for prescribing subsidised ferric carboxymaltose in the community8
Ferric carboxymaltose is contraindicated in patients:9
- With anaemia not due to iron deficiency, e.g. megaloblastic anaemia, anaemia of chronic disease without iron deficiency,
glucose-6-phosphate dehydrogenase (G6PD) deficiency
- With evidence of iron overload or disturbances in utilisation of iron, e.g. haemochromatosis, thalassaemia
- Aged under 14 years; no studies have been conducted in patients of this age
- In the first trimester of pregnancy; it is recommended to consult with an obstetrician regarding the risks and benefits
of treatment at other stages of pregnancy
- With a previous hypersensitivity to other parenteral iron products
Compared to oral iron supplementation, intravenous formulations have lower rates of gastrointestinal adverse effects
such as constipation and diarrhoea, but are associated with injection site reactions.10, 11
The most common adverse effects of intravenous ferric carboxymaltose observed in clinical trials are (with % incidence):10
- Hypophosphataemia (2–27% – see “Follow-up” below)
- Nausea (3–7%)
- Hypertension (1–4%) *
- Injection site reactions (1–2%)
- Headache (1%)
- Dizziness (1%)
- Increased liver enzymes (1%)
* Most reports of hypertension involved increases in blood pressure immediately following
administration which resolved within 30 minutes
Advise patients that they may experience delayed adverse effects one to two days following administration, such as mild
fever, headache, arthralgia or myalgia.12
Hypersensitivity is rare, but practices should take precautions
Rates of serious anaphylactoid reactions with ferric carboxymaltose are estimated to be approximately 0.1%.1, 12 Although
the risk of hypersensitivity is low, practices that administer ferric carboxymaltose should have staff trained to evaluate
and manage anaphylactoid reactions and resuscitation facilities available.13 The use of a “test dose” to assess
the potential for anaphylaxis is not recommended as it does not reliably predict how a patient will react to a full dose.13 Hypersensitivity
reactions can also occur in patients who have previously tolerated parenteral iron products. Patients should remain in
the practice for observation for 30 minutes following administration of ferric carboxymaltose.
Ferric carboxymaltose can be given either as a slow intravenous undiluted injection or as an infusion diluted in sterile
0.9% saline.* In both cases, an intravenous cannula should be fitted and initially flushed with 10 mL of saline in order
to check placement and possible leakage; ferric carboxymaltose should not be given as an intramuscular injection and may
cause irritation and skin discolouration if extravasation occurs.9, 12
It is recommended that the Ganzoni formula is used to calculate the iron dose to be administered.9 An
alternative, simpler, method is to determine the required elemental iron dose using the patient’s haemoglobin levels
and body weight (Table 1). However, this method has only been evaluated in one trial.9 If
patients require more than 1000 mg of elemental iron, the dose must be administered over two sessions at least seven
days apart.1
* Sterile 0.9% saline is the only diluent that should be used; ferric carboxymaltose should not be diluted for infusion
in other solutions.
Table 1: Elemental iron requirements using a simplified formula9, 14
| Haemoglobin level |
Body weight |
| 35–70 kg |
≥ 70 kg |
| ≥ 100 g/L |
1000 mg |
1500 mg |
| 70–100 g/L |
1500 mg |
2000 mg |
The Ganzoni formula
Cumulative elemental iron dose* = body weight [kg] × (target haemoglobin † – actual haemoglobin [g/L]) × 0.24 + Iron stores† [mg]
* Round the cumulative elemental iron dose down to the nearest 100 mg for patients with a body weight ≤ 66 kg, and round
it up to the nearest 100 mg for patients with a body weight > 66 kg
† Target haemoglobin (Hb) and iron store values for the calculation:
| Patient’s body weight |
Target haemoglobin |
Estimated iron stores |
| < 35 kg |
130 g/L |
Calculate at: 15 mg/kg |
| ≥ 35 kg |
150 g/L |
500 mg |
Example: A patient weighing 58 kg, with a current haemoglobin level of 89 g/L would have a target Hb of 150 g/L
(body weight is over 35 kg) and estimated iron stores of 500 mg. Therefore the calculation
for their elemental iron dose is:
58 [kg] × (150 – 89 [g/L]) × 0.24 + 500 [mg] = 1349 mg of elemental iron; round down to 1300 mg as body weight is ≤
66 kg (administered in two sessions at least seven days apart)
Calculators are available online for estimating iron requirements using the Ganzoni formula, e.g.
http://www.jackofallorgans.com/iron
How to administer ferric carboxymaltose
As a slow intravenous undiluted injection:9
1. Flush cannula with 10 mL of saline
2. One 500 mg vial can be drawn up undiluted into a 10 mL syringe*; an 18 gauge needle is recommended for drawing ferric
carboxymaltose up from the vial
3. Use a 20 or 22 gauge needle for slow injection
4. For doses:
- Up to 500 mg, aim for a rate of 100 mg (2 mL) per minute, i.e. a 500 mg vial should take five minutes to inject
- From 500–1000 mg, inject over a period of 15 minutes
5. Ask patients to remain in the clinic for 30 minutes to monitor for hypersensitivity reactions9, 13
As a drip infusion:9
1. Flush cannula with 10 mL of saline
2. Dilute ferric carboxymaltose in 0.9% sterile saline; do not dilute below 2 mg iron/mL (not including the volume of
the ferric carboxymaltose solution). Infuse over an appropriate timeframe up to 15 minutes.
Suggested volumes of dilution and administration times:
| Elemental iron dose (corresponding volume of ferric carboxymaltose*): |
Dilute into a 0.9% saline bag of maximum volume: |
Administer by drip infusion over: |
| 100–200 mg (2–4 mL) |
50 mL |
3 minutes |
| 200–500 mg (4–10 mL) |
100 mL |
6 minutes |
| 500–1000 mg (10–20 mL) |
250 mL |
15 minutes |
3. Ask patients to remain in the clinic for 30 minutes in case of hypersensitivity reactions9, 13
* Ferric carboxymaltose contains 50 mg/mL elemental iron; subsidised preparation is a 500 mg/10 mL vial
Normalisation of haemoglobin level and red blood cell indices is typically the aim of treatment in patients with iron
deficiency anaemia without co-morbidities. Check full blood count monthly until haemoglobin levels have normalised, with
blood samples taken at least one week after ferric carboxymaltose administration.3, 12 Testing can be reduced
to three monthly once results have stabilised.3
The optimal frequency of monitoring and targets for treatment may differ for patients with co-morbidities. In patients
with chronic kidney disease, for example, the aim is often to maintain transferrin saturation level above 20% and serum
ferritin level above 100 micrograms/L, with the target haemoglobin level depending on the stage of chronic kidney disease
and other patient characteristics.15
Re-treatment with ferric carboxymaltose is not usually needed, but may be required for some patients, especially those
with a co-morbidity contributing to their iron deficiency anaemia. For example, half of patients with inflammatory bowel
disease who are treated for iron deficiency anaemia have a recurrence within 10 months.4
Clinicians should be aware that transient hypophosphataemia may occur after administration of ferric carboxymaltose.
However, this is typically asymptomatic, does not require investigation or treatment and resolves after an average of
three weeks.10, 16, 17 Case reports suggest that in rare instances patients can develop severe or symptomatic
hypophosphataemia after receiving ferric carboxymaltose. This has typically occurred after multiple administrations, e.g.
due to a chronic condition such as active inflammatory bowel disease, or in patients with co-morbidities which affect
phosphate homeostasis such as a hyperparathyroidism or vitamin D deficiency.18, 19 If there is clinical suspicion
of symptomatic hypophosphataemia, e.g. patients have bone pain, muscle weakness or altered mental state, then testing
of phosphate levels, correction of low levels and consideration of an alternative treatment for iron deficiency anaemia
may be necessary.10
For further information on diagnosing iron deficiency anaemia, see:
www.bpac.org.nz/BT/2013/September/investigating-anaemia.aspx
