Published: 15th March 2024
Key practice points:
- Anticholinergic burden refers to the cumulative effect of taking medicines with anticholinergic activity
- Frequently prescribed anticholinergic medicines include antidepressants (e.g. amitriptyline), antiemetics (e.g. cyclizine), antipsychotics (e.g. chlorpromazine) and oxybutynin (for urinary urgency and incontinence)
- There are a range of adverse effects that can occur with anticholinergic medicines, and these are generally dose dependent:
- Centrally mediated effects include drowsiness, restlessness and impaired concentration. People may experience severe agitation, hallucinations and delirium with higher doses.
- Peripheral effects include dry mouth, pupillary dilation, decreased sweating, urinary retention, vasodilation, tachycardia, constipation
- Long-term use of anticholinergic medicines is associated with reduced mobility, cognitive impairment and the development of dementia (causation has not been definitively established)
- Older people are at higher risk of anticholinergic burden due to age-related changes in physiology, and increased likelihood of multiple morbidities requiring management with anticholinergic medicines
- Anticholinergic burden should be assessed in any patient who is prescribed an anticholinergic medicine and may be at higher risk of anticholinergic adverse effects based on their age or frailty status. Specific opportunities for assessment include the initiation of a new medicine, following a hospital discharge or if the patient presents with symptoms that may have an anticholinergic origin, e.g. deteriorating oral health, a recent fall.
- Consider referring patients at higher risk of medicine-related harm to a clinical pharmacist for a medicines assessment
- If a high anticholinergic burden is identified, dose reduction, deprescribing or switching treatments are possible courses of action, depending on the clinical scenario and the patient’s therapeutic goals and treatment preferences
- Prioritise non-pharmacological interventions to reduce the required dose of, or overall need for, anticholinergic medicines
- If an anticholinergic medicine is required in an older person, choose an option with the least anticholinergic activity, prescribe at the lowest effective dose for the shortest possible duration, discuss adverse effects and when to seek medical attention
- Regularly review the patient to assess treatment efficacy and presence of adverse effects
The neurotransmitter, acetylcholine, binds to both muscarinic and nicotinic receptors, resulting in classical cholinergic effects, such as increased salivation, lacrimation, bronchial secretions and central nervous system arousal.1 Anticholinergic medicines act by competitively blocking acetylcholine binding sites on muscarinic* receptors, counteracting these effects. The terms anticholinergic antagonist and antimuscarinic antagonist are often used interchangeably.1 Medicines with anticholinergic activity include antidepressants, antihistamines, antipsychotics and medicines to treat urinary urgency and incontinence (Table 1).1 In some cases, the anticholinergic antagonist action is used therapeutically, e.g. oxybutynin for urinary incontinence or benzatropine for Parkinson’s disease.1 However, many commonly prescribed medicines also have undesired anticholinergic effects unrelated to their therapeutic actions, e.g. amitriptyline, promethazine, quetiapine.1 People who are prescribed multiple medicines with anticholinergic activity are at increased risk of adverse effects, and this cumulative anticholinergic influence is referred to as the anticholinergic burden, although it can occur with just one anticholinergic medicine.1
* Medicines that act as antagonists at nicotinic acetylcholine receptors are not generally used in a community setting, e.g. neuromuscular blocking agents for general anaesthesia2
Table 1. Examples of prescription and over-the-counter medicines with anticholinergic activity.3-6 N.B. This list is not exhaustive and should be used a general guide only as there is inconsistency in anticholinergic rankings between sources. Any medicine with any level of anticholinergic activity should be used with caution in patients susceptible to the adverse effects, especially if used in combination.
| Class |
Medicines with anticholinergic activity |
| High anticholinergic activity |
Mixed evidence for high anticholinergic activity* |
Moderate to low anticholinergic activity |
Antidepressants
SSRIs and SNRIs |
|
Paroxetine |
Citalopram, escitalopram, fluoxetine, sertraline, venlafaxine |
TCAs and other |
Amitriptyline, clomipramine, imipramine |
Nortriptyline |
Dosulepin, mirtazapine, moclobemide |
Antiepileptics |
|
|
Carbamazepine |
Antihistamine |
Chlorphenamine, dexchlorpheniramine, diphenhydramine, doxylamine, promethazine |
|
Cetirizine, fexofenadine, loratadine |
Antinausea |
Meclozine (meclizine) |
|
Cyclizine, prochlorperazine |
Antipsychotics |
Chlorpromazine, levomepromazine |
Clozapine, olanzapine, quetiapine |
Amisulpride, aripiprazole, haloperidol, lithium, risperidone, ziprasidone |
Benzodiazepines |
|
Alprazolam |
Clobazam, clonazepam, diazepam, lorazepam, oxazepam, temazepam |
Bronchodilators (antimuscarinic) |
Ipratropium |
|
Glycopyrronium, tiotropium, umeclidinium |
Cardiac medicines |
Atropine |
Digoxin |
|
Diuretics |
|
Furosemide |
|
Gabapentinoids |
|
|
Gabapentin, pregabalin |
Gastrointestinal medicines |
Hyoscine (scopolamine) |
|
Domperidone, loperamide, metoclopramide |
Skeletal muscle relaxants |
Orphenadrine |
|
|
Opioids |
|
|
Codeine, dihydrocodeine, fentanyl, methadone, morphine, oxycodone, pethidine, tramadol |
Parkinson’s medicines |
Benzatropine, procyclidine |
|
Amantadine, levodopa |
Urinary urgency and incontinence medicines |
Oxybutynin, solifenacin |
|
|
SNRI = serotonin and noradrenaline reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant
*High anticholinergic activity according to some, but not all anticholinergic rating scales6
Acute and long-term adverse effects of anticholinergic medicines
The adverse effect profile of anticholinergic medicines is diverse (Figure 1). This can be explained by the wide distribution of muscarinic receptors across different organ systems and the lack of selectivity anticholinergic medicines have for specific muscarinic receptor subtypes.2 The cumulative effect of taking concomitant anticholinergic medicines further increases the risk of adverse effects, particularly in older people.1
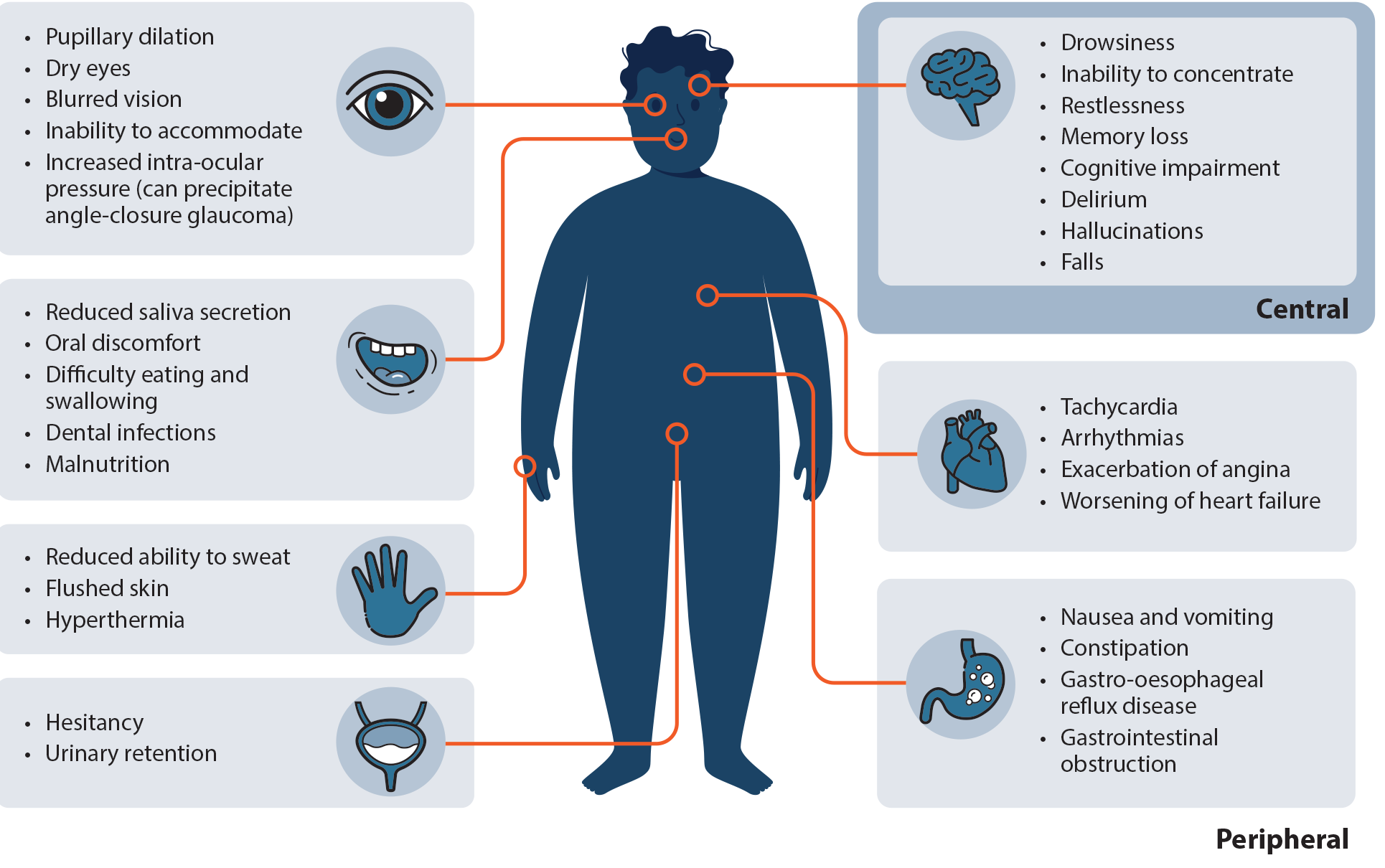
Figure 1. Overview of adverse anticholinergic effects.1, 4, 5
Acute effects act on the peripheral or central nervous systems
Acute adverse anticholinergic effects can be classified as either central or peripheral and their severity is generally dose dependent (Table 2).1, 7 Central effects are a direct result of reduced acetylcholine activity in the brain and are determined by a medicine’s ability to penetrate the blood brain barrier and its affinity for muscarinic receptors.1, 5 Peripheral changes relate to anticholinergic effects on muscarinic receptors reducing secretions and muscle contraction outside the central nervous system.1 In combination, central and peripheral anticholinergic adverse effects (e.g. drowsiness, impaired concentration and blurred vision) can increase the risk of falls and therefore, fractures in older people.5 Emerging evidence also points to a higher risk of acute cardiovascular events in the period immediately following an increase in anticholinergic burden, i.e. initiating a new medicine with anticholinergic activity.8
Table 2. Acute central and peripheral adverse anticholinergic effects.1, 3
|
Central anticholinergic symptoms
|
Peripheral anticholinergic symptoms
|
- Drowsiness
- Restlessness
- Impaired concentration
- Agitation
- Hallucinations
- Delirium
- Cognitive dysfunction (e.g. temporary dementia-like state)
|
- Constipation (due to reduced peristalsis)
- Pupillary dilation and blurred vision
- Decreased salivary gland secretion (resulting in dry mouth)
- Anhidrosis (decreased sweating)
- Tachycardia
- Urinary retention
- Cutaneous vasodilation
|
The mnemonic “red as a beet (cutaneous vasodilation), dry as a bone (decreased salivary gland secretion), blind as a bat (pupillary dilation and blurred vision), mad as a hatter (altered mental status), hot as a hare (anhidrosis leading to temperature elevation) and full as a flask (urinary retention)” was historically used to remember anticholinergic symptoms.
Long-term effects with ongoing use of anticholinergic medicines
Increased anticholinergic burden in older people is associated with a decline in both cognitive functioning and mobility.9–11 A 2021 Cochrane review concluded that anticholinergic medicine use in older people without current cognitive impairment was associated with an increased risk of future cognitive impairment and dementia.10 When adjusted for sex, age and co-morbidities, the relative risk may be more than twice that of older people who do not take anticholinergic medicines.10 This effect may also increase as a person’s anticholinergic burden increases; older people with a “severe” anticholinergic burden were reported to have a 227% increased risk for developing cognitive impairment and dementia.10 However, the quality of evidence included in this study was considered to be low, and was insufficient to determine whether anticholinergic medicines have a direct causal role in cognitive impairment or dementia.10 It is also unclear if these long-term effects are reversible following cessation of anticholinergic medicines, and it may be patient-specific.10 Evidence linking anticholinergic medicines use to further cognitive decline in people with pre-existing cognitive impairment or dementia is inconsistent.12
Chronic effects of increased anticholinergic burden are not limited to the central nervous system. Long-term reductions in salivary gland secretion (xerostomia) can lead to increased rates of oral infections and tooth decay, compromised taste, difficulty swallowing, nutritional deficiencies and halitosis.13 The fitting of partial or complete dental protheses may also be difficult in people with a chronic dry mouth.13
Medicines interactions
Medicines interactions may be more significant in older people who are prescribed anticholinergic medicines. The concomitant use of anticholinergic medicines and sedatives (e.g. zopiclone) can cause more pronounced sedation, increasing the risk of falls, reducing appetite and contributing to cognitive impairment.14 Anticholinergic medicines and cholinesterase inhibitors (e.g. donepezil), prescribed for dementia, have opposing pharmacological actions.15 The concomitant use of these medicines reduces their therapeutic efficacy and may negatively affect cognitive function.15
For further information on specific medicines interactions, refer to the NZF interactions checker: www.nzf.org.nz
Anticholinergic medicines and older people
In New Zealand, more than 40% of people aged over 65 years are exposed to medicines with anticholinergic activity.16 This group is at increased risk of acute adverse anticholinergic effects (e.g. dry mouth, blurred vision, urinary retention and drowsiness) as well as long-term effects such as impaired cognitive function and reduced mobility which may result in falls.1 Factors that can influence anticholinergic burden in older people include:
- Inter-individual variability (determined by genetics, sex and ethnicity)1
- Age-related physiological changes such as increased blood brain barrier permeability and reduced hepatic or renal function can result in higher serum concentrations of anticholinergic medicines1
- Multiple health conditions that require different medicines for management; a population study found that in 2018, 10% of people in New Zealand were prescribed five or more medicines and three-quarters of those people were aged over 60 years17
- Misinterpretation of anticholinergic adverse effects in older people as normal signs of ageing, e.g. cognitive or memory impairment, loss of balance.18 These adverse effects may not be reported or investigated during an assessment.18
- Initiating new medicines to manage the adverse effects caused by existing medicines, i.e. the “prescribing cascade”5
Primary care clinicians are ideally placed to identify people at risk of a higher anticholinergic burden. Consider assessing anticholinergic burden in any patient who is prescribed at least one anticholinergic medicine (Table 1) and may be at higher risk of anticholinergic adverse effects based on their age or frailty status; this may be done opportunistically during a routine appointment. Patients presenting with a history of falls or any symptoms with a potential anticholinergic cause may also warrant assessment, e.g. deteriorating oral health. Other situations that may prompt consideration of anticholinergic burden in an older or frail person include initiation of a new medicine or review after discharge from hospital.
Checklist:
- Is the patient prescribed an anticholinergic medicine?
- Is the patient at risk of anticholinergic adverse effects based on their age or frailty status?
- Is the patient experiencing any symptoms or adverse effects that could be related to their use of anticholinergic medicines?
If the answer to any of these questions is yes, then an assessment of the patient’s anticholinergic burden should be undertaken. The assessment may involve:
- A comprehensive medicines review to determine what medicines the patient is currently taking, including any prescribed by other clinicians and any over-the-counter products or alternative treatments. A medicines reconciliation or “brown bag review” may be required – ask the patient to bring all medicines they are currently using with them to the appointment.
- Establishing the reason each medicine was prescribed. Also ask about adherence (e.g. is the prescribed medicine actually being taken?) and any adverse effects that may be related to a medicine.
- Identifying which medicines have anticholinergic activity and could be contributing to the patient’s anticholinergic burden. Table 1 can be used as a general guide to the most commonly prescribed anticholinergic medicines; the properties of other medicines can be checked using the NZF. Tools have been developed to aid the quantification of anticholinergic burden, however, none are specifically recommended for New Zealand primary care as they are often not readily accessible online and there is potential for differing results depending on the anticholinergic scale the tool was based on (see: “Anticholinergic scales may be helpful in assessing anticholinergic burden”).
Anticholinergic scales may be helpful in assessing anticholinergic burden
Various clinical metrics have been developed to identify and measure anticholinergic burden, e.g. Anticholinergic Activity Scale (AAS), Anticholinergic Burden Classification (ABC) scale, Anticholinergic Drug Scale (ADS), Anticholinergic Risk Scale (ARS) and Drug Burden Index (DBI).5, 19 These scales provide lists of medicines with anticholinergic activity that are categorised based on anticholinergic potency.19 Some of the scales also incorporate daily and cumulative medicine exposure when assessing anticholinergic burden.19 A cumulative score can be calculated to quantify anticholinergic burden.19 In many cases, higher scores are associated with an increased likelihood of adverse anticholinergic effects.19
The most appropriate scale for use in New Zealand primary care setting is unclear. The determination of anticholinergic effect is inconsistent between the scales, with some constructed using serum anticholinergic activity as a measure while others are based on pharmacological principles or expert opinion.19 For instance, the anticholinergic activity of quetiapine ranges from zero (no anticholinergic effect) in the ADS through to three (large anticholinergic effect) in the ABC scale.19 Further limitations include the assumption that the anticholinergic activity of different medicines is linear and additive, the applicability of included medicines to the New Zealand health system, as well as not accounting for variability in individual susceptibility to anticholinergic effects, e.g. impaired renal or hepatic function, other co-morbidities.3, 19 In practice, anticholinergic scales may be beneficial to determine if a person is at risk of adverse anticholinergic effects but not to precisely quantify their anticholinergic burden or as the sole basis for clinical decisions.
N.B. Links provided for anticholinergic scales provide further information but in most cases the scales themselves are not readily available online.
The Health Quality and Safety Commission has included the following resources in the Appropriate Prescribing Toolkit:
- The American Geriatrics Society (AGS) Beers Criteria (the Beers list) – an internationally recognised list of medicines that are potentially inappropriate for use in older patients. The list was developed using a Delphi consensus and was updated most recently in 2023.
- Drug burden index (DBI) – a tool to quantitatively measure an individual’s exposure to anticholinergic and sedative medicines. Higher DBI scores are associated with poorer health outcomes in older people.
- Anticholinergic Cognitive Burden Scale (ACB)/Anticholinergic Risk Scale (ARS) – both tools categorically rank medicines with anticholinergic activity and can be used to predict increased anticholinergic burden in older people. ACB or ARS scores of three or greater are indicative of clinically relevant anticholinergic burden and higher scores are associated with adverse effects.
An online ACB scale calculator is available. Medicines are entered into the calculator and assigned a score of 1, 2 or 3. Using a combination of the ACB scale and the German Anticholinergic Burden Scale (GABS), medicines with definite anticholinergic activity are assigned a score of 2 or 3. Medicines with possible anticholinergic activity are assigned a score of 1. Medicines not included in the calculator are assumed to have a score of 0. A total ACB score ≥ 3 indicates that the medicine or medicines combination entered into the calculator is putting the person at increased risk of adverse anticholinergic effects and switching or deprescribing may be appropriate.
N.B. The medicines included in this resource may not reflect all anticholinergic medicines available in New Zealand.
Over-the-counter medicines may have high anticholinergic activity
Always ask about use of over-the-counter medicines and complementary and alternative medicines when considering anticholinergic burden.
Antihistamines are readily available without a prescription and have the potential to significantly affect anticholinergic burden. They are commonly found in medicines used to manage allergies, motion sickness, cough and cold and as a short-term treatment for insomnia. For example, chlorphenamine, diphenhydramine and doxylamine, found in preparations for cough and cold or insomnia, and promethazine and meclozine, used to treat symptoms of motion sickness, are considered to have high anticholinergic activity.6
Hyoscine is an antimuscarinic medicine available over the counter for the treatment of gastrointestinal muscle spasms (hyoscine butylbromide, e.g. Gastro-Soothe; tablet) and motion sickness (hyoscine hydrobromide, e.g. Scopoderm; transdermal patch); both formulations may contribute to anticholinergic burden. In 2023, the United Kingdom Medicines and Healthcare products Regulatory Agency (MHRA) released a drug safety update regarding the potential for significant anticholinergic adverse effects as a result of inappropriate use of hyoscine hydrobromide transdermal patches, e.g. cutting or applying multiple patches, continuous use without a break, long-term use.20
Some complementary and alternative medicines (CAMs) have anticholinergic activity and could interact with prescribed medicines, e.g. herbal remedies derived from Datura or other nightshade plants.21 If the patient is using CAMs, further investigation may be warranted to identify if any of the ingredients have anticholinergic activity. Encourage patients to bring product containers (or pictures of the container) to a medicines review.
Pharmacists must also be vigilant in considering anticholinergic burden when supplying over-the-counter medicines and CAMs, i.e. ask about other medicines the patient is taking.
Refer for a pharmacist medicines review
Medicine review services are available in some areas (refer to local HealthPathways), or large practices may have clinical pharmacists on staff. Increasing evidence indicates that pharmacist-led medicine reviews and deprescribing recommendations are an effective and underutilised tool to reduce individual anticholinergic burden in older people.22, 23 Primary care prescribers can refer patients at higher risk of medicines-related harm to a clinical pharmacist for a medicines review of their prescription and over-the-counter medicines.
Once a high anticholinergic burden has been established, a management plan, made in consultation with the patient, should be implemented to reduce the number of anticholinergic medicines they are taking. Potential strategies for this include deprescribing or reducing the dose or switching to another medicine if possible. At all times, non-pharmacological strategies should be reinforced as this may reduce the required dose of the medicine, or the need for the medicine at all (see: “Optimise non-pharmacological management strategies”).
Optimise non-pharmacological management strategies
Non-pharmacological interventions may help to reduce the required dose of, or overall need for, medicines with anticholinergic activity while still managing the condition.24 Examples of non-pharmacological management strategies include:4, 24
- Urinary incontinence: pelvic floor exercises, regular bladder emptying, incontinence aids and avoiding excessive fluid intake, reducing caffeine and alcohol
- Depression and insomnia: sleep hygiene, minimising alcohol consumption, following a healthy diet and reducing stress, cognitive behavioural therapy and behavioural activation, identify and deprescribe (or reduce the dose of) medicines that disrupt sleep, e.g. corticosteroids, beta blockers, if possible
- Pain conditions: increasing daily activity, regular exercise, physiotherapy, transcutaneous electrical nerve stimulation (TENS), behavioural distraction techniques, e.g. guided imagery
Deprescribing anticholinergic medicines
Deprescribing may be an option for patients who are identified as having a high anticholinergic burden,4 especially if the potential harms of any prescribed medicines outweigh the benefits for the patient at their current stage of life. Factors to consider when deciding whether a medicine should be reduced or stopped include:24, 25
- Is the original indication for this medicine still relevant?
- Could any reported adverse effects be caused by this medicine?
- Does the prescribed medicine still provide benefit for the patient and does that outweigh the risk of potential adverse effects?
- What are the patient’s therapeutic goals at their stage of life?
- Has there been any change in the patient’s clinical condition that may render the currently prescribed medicine or dose inappropriate?
- Is there another medicine for the relevant indication that may be more appropriate for this patient?
 Best practice tip: Deprescribe one medicine at a time to simplify the process for the patient, and allow recognition and correct attribution of discontinuation symptoms.26
Best practice tip: Deprescribe one medicine at a time to simplify the process for the patient, and allow recognition and correct attribution of discontinuation symptoms.26
Shared decision-making is an important part of the deprescribing process
Patients may be hesitant to reduce their dose or stop taking a medicine for various reasons, e.g. they attribute improvement in their quality of life to this medicine, are fearful of the original symptoms returning, have previously experienced withdrawal symptoms, were told “you will need to take this for the rest of your life”.26 In this situation, the clinician should ask about the patient’s therapeutic goals and treatment preferences before discussing the potential risks and benefits of continuing the anticholinergic medicine. Patient engagement is a key driver for successful deprescribing, therefore it may be beneficial to explain your rationale, e.g. the medicine is no longer needed for the reason you started it in the first place, it increases your risk of falls and it might make your memory worse.26
For further information on deprescribing in primary care, see: bpac.org.nz/2018/stopping.aspx
A gradual taper is necessary when deprescribing medicines with anticholinergic activity
Medicines with anticholinergic activity should be gradually tapered as they are withdrawn, to avoid cholinergic discontinuation syndrome (see: “Cholinergic discontinuation syndrome”).3, 5 A general “rule of thumb” for tapering anticholinergic medicines is to reduce the prescribed dose by 25 – 50% over a period of one to four weeks.24 Patients who have been prescribed anticholinergic medicines for extended periods (e.g. antipsychotics) may require a more gradual taper.27 Alternate day dosing may be beneficial in situations where available medicine strengths are not appropriate for tapering.24
Close monitoring is required over the tapering period for anticholinergic withdrawal symptoms (over the first one to three days) and recurrence of symptoms associated with the condition originally being treated (after approximately seven days).24 Patients who develop withdrawal symptoms or a reoccurrence of their original symptoms should restart the medicine at the lowest tolerated dose and reattempt a slower tapered reduction after 6 – 12 weeks.24
A tapering calculator to assist with planning and prescribing a tapering schedule is available at: www.jackofallorgans.com/tapering/
For further information on stopping medicines in older people, see: bpac.org.nz/BPJ/2010/April/stopguide.aspx
Cholinergic discontinuation syndrome
Cholinergic discontinuation syndrome (also called cholinergic rebound) can develop within a few days of abruptly stopping anticholinergic medicines, and can persist for six to eight weeks.3, 4 The risk of withdrawal symptoms is higher with antidepressants, antipsychotics, benzodiazepines, opioids and sedating antihistamines compared to anticholinergic medicines for urinary incontinence and non-sedating antihistamines.4 Commonly reported symptoms of cholinergic discontinuation syndrome are nausea, vomiting and diarrhoea, lacrimation, dizziness, anxiety, sweating, urinary urgency and insomnia, i.e. cholinergic effects, the severity of which is variable.3–5 This likely results from the sudden availability, and subsequent overstimulation, of acetylcholine receptors. Restarting the anticholinergic medicine at the lowest effective dose may be required in patients who develop severe withdrawal symptoms, e.g. tachycardia, orthostatic hypotension, fever, or symptoms significantly affecting their quality of life, e.g. sweating, anxiety, insomnia.4 A more gradual taper can be attempted after 6 – 12 weeks.24 The potential risks of discontinuing an anticholinergic medicine should be discussed with the patient prior to a decision being made to modify their medicines regimen.3
Switching to another medicine with lower anticholinergic activity
Ideally, medicines with high anticholinergic activity should be avoided in older people unless absolutely necessary.1 Older patients who still require pharmacological management may benefit from switching to medicines with lower or no anticholinergic activity, if available (Table 3).1 The decision to change to another medicine should be made after a discussion with the patient regarding the benefits and risks of switching, e.g. reduced adverse effects, potential reduction in clinical effect compared to original medicine. When changing medicines, gradual dose tapering of the original anticholinergic medicine may be required to limit withdrawal symptoms (see: “A gradual taper is necessary when deprescribing medicines with anticholinergic activity”).
Table 3. Examples of medicine “swaps” for people with high anticholinergic burden.1, 3–5, 28, 29
Medicines to avoid if possible |
Medicines to select instead
N.B. In some cases a preferred option may still have some anticholinergic activity. |
Allergy medicines |
|
Chlorphenamine, dexchlorpheniramine, promethazine |
Cetirizine, loratadine, intranasal corticosteroids |
Antidepressants |
|
Amitriptyline, nortriptyline, paroxetine |
Sertraline or venlafaxine |
Antinausea medicines |
|
Prochlorperazine |
Domperidone, metoclopramide, ondansetron (may be inappropriate in older people due to risk of adverse effects, e.g. prolonged QT interval, tardive dyskinesia) |
Antipsychotics |
|
Chlorpromazine, clozapine, doxepin, levomepromazine |
Amisulpride, aripiprazole, haloperidol, risperidone |
Anxiolytics |
|
Diazepam |
Lorazepam (ideally avoid prescribing a benzodiazepine for this indication) |
Hypnotics |
|
Diazepam, temazepam, promethazine, diphenhydramine |
Non-pharmacological options are preferred, e.g. sleep hygiene, or short-term use of melatonin or zopiclone |
Neuropathic pain medicines |
|
Amitriptyline, nortriptyline |
Gabapentin, pregabalin (N.B. Limited evidence for benefit; see: Gabapentinoids: when and how should they be prescribed?) |
Urinary urgency and incontinence medicines |
|
Oxybutynin, solifenacin* |
No other funded medicines available in New Zealand (non-anticholinergic options are available in other countries). Consider non-pharmacological options, e.g. incontinence aids and avoiding excessive fluid intake. |
*In practice, solifenacin is often favoured over oxybutynin as it may be more effective, however, anticholinergic adverse effects are just as likely.
There are some clinical situations where the initiation or continuation of anticholinergic medicines is appropriate, even in older or frail high-risk patients, e.g. tricyclic antidepressants for the management of neuropathic pain, clozapine treatment for refractory schizophrenia.3, 7 If required, medicines with anticholinergic activity should be prescribed at the lowest effective dose, for the shortest possible duration.1 The challenge for clinicians is finding a balance between effectively treating the condition and avoiding anticholinergic adverse effects.
Before prescribing medicines with anticholinergic activity to older people, it is useful to establish their baseline cognitive level.3 This may aid early recognition of cognitive deterioration and facilitate timely deprescribing. Anticholinergic medicines may also worsen symptoms in people with co-morbid conditions, e.g. glaucoma, tachyarrhythmias, benign prostatic hyperplasia and constipation.2 Consider establishing a baseline for these conditions and closely monitor for clinical deterioration.3
Once an anticholinergic medicine is prescribed, ensure the patient knows what adverse effects to expect and when to seek advice. Regularly monitor treatment efficacy along with the patient’s status, especially if the medicine is continued long-term.
Checklist when prescribing anticholinergic medicines in older people:
- Review the current medicine regimen before adding a medicine with anticholinergic activity
- Choose medicines with limited anticholinergic activity
- Consider a short duration of the lowest effective dose
- Conduct a baseline cognitive assessment
- Provide patients and carers with clear instructions regarding what adverse effects to look out for and when to seek medical attention if they develop
- Re-evaluate the patient regularly if treatment is required for an extended period





